The time of maximum effect for model selection in pharmacokinetic-pharmacodynamic analysis applied to frusemide
- PMID: 9489596
- PMCID: PMC1873996
- DOI: 10.1046/j.1365-2125.1998.00637.x
The time of maximum effect for model selection in pharmacokinetic-pharmacodynamic analysis applied to frusemide
Abstract
Aims: Both indirect-response models and effect-compartment models are used to describe the pharmacodynamics of drugs when there is a delay in the time course of the pharmacological effect in relation to the concentration of the drug. The aim of this study was to investigate whether the time of maximum response after single-dose administration at different dose levels could be used to distinguish between these models and to select the most appropriate pharmacokinetic-pharmacodynamic model for frusemide.
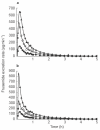
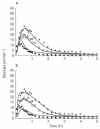
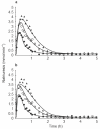
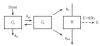
Methods: Three doses of frusemide, 10, 25 and 40 mg were given as rapid intravenous infusions to five healthy volunteers. Urine samples were collected for 5 h after dosing. Volume and sodium losses were isovolumetrically replaced with an intravenous rehydration fluid. Diuresis and natriuresis were modelled for all three doses simultaneously, applying both an indirect-response model and an effect-compartment model with the frusemide excretion rate as the pharmacokinetic input.
Results: The observed time of maximum diuretic and natriuretic response significantly increased with dose. This increase was well predicted by the indirect-response model, whereas the modelling with the effect-compartment model led to a poor prediction of the peaks. There was no difference between the observed and predicted time of maximum diuretic and natriuretic response using the indirect-response model, whereas the time of maximum response predicted by the effect-compartment model was significantly earlier than the time observed for the 25 mg (P < 0.05) and 40 mg (P < 0.05) doses.
Conclusions: The time of maximum response to frusemide was better described using an indirect-response model than an effect-compartment model. Studying the time of maximum response after administration of different single doses of a drug may be used as a selective tool during pharmacokinetic-pharmacodynamic modelling.
Figures
References
-
- Levy G. Mechanism-based pharmacodynamic modelling. Clin Pharmacol Ther. 1994;56:356–358. - PubMed
-
- Wakelkamp M, Alván G, Gabrielsson J, Paintaud G. Pharmacodynamic modelling of furosemide tolerance after multiple intravenous administration. Clin Pharmacol Ther. 1996;60:75–88. - PubMed
-
- Odlind B, Beermann B. Renal tubular secretion and effects of furosemide. Clin Pharmacol Ther. 1980;27:784–790. - PubMed
-
- Holford NHG. Parametric models of the time course of drug action. In: van Boxtel CJ, Holford NHG, Danhof M, editors. The in vivo study of drug action. Amsterdam: Elsevier; 1992. pp. 61–70.
-
- Holford NHG, Sheiner LB. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981;6:429–453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

