A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis
- PMID: 9490714
- PMCID: PMC2132686
- DOI: 10.1083/jcb.140.5.991
A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis
Abstract
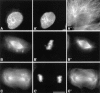
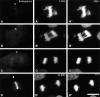
INCENP is a tightly bound chromosomal protein that transfers to the spindle midzone at the metaphase/anaphase transition. Here, we show that an INCENP truncation mutant (INCENP382-839) associates with microtubules but does not bind to chromosomes, and coats the entire spindle throughout mitosis. Furthermore, an INCENP truncation mutant (INCENP43-839) previously shown not to transfer to the spindle at anaphase (Mackay, A.M., D.M. Eckley, C. Chue, and W.C. Earnshaw. 1993. J. Cell Biol. 123:373-385), is shown here to bind chromosomes, but is unable to target to the centromere. Thus, association with the chromosomes, and specifically with centromeres, appears to be essential for INCENP targeting to the correct spindle subdomain at anaphase. An INCENP truncation mutant (INCENP1-405) that targets to centromeres but lacks the microtubule association region acquires strong dominant-negative characteristics. INCENP1-405 interferes with both prometaphase chromosome alignment and the completion of cytokinesis. INCENP1-405 apparently exerts its effect by displacing the endogenous protein from centromeres. These experiments provide evidence of an unexpected link between this chromosomal protein and cytokinesis, and suggest that one function of INCENP may be to integrate the chromosomal and cytoskeletal events of mitosis.
Figures
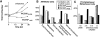






References
-
- Bernat RL, Delannoy MR, Rothfield NF, Earnshaw WC. Disruption of centromere assembly during interphase inhibits kinetochore morphogenesis and function in mitosis. Cell. 1991;66:1229–1238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

