AP-2/Eps15 interaction is required for receptor-mediated endocytosis
- PMID: 9490719
- PMCID: PMC2132690
- DOI: 10.1083/jcb.140.5.1055
AP-2/Eps15 interaction is required for receptor-mediated endocytosis
Abstract
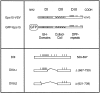
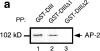
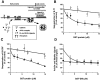
We have previously shown that the protein Eps15 is constitutively associated with the plasma membrane adaptor complex, AP-2, suggesting its possible role in endocytosis. To explore the role of Eps15 and the function of AP-2/Eps15 association in endocytosis, the Eps15 binding domain for AP-2 was precisely delineated. The entire COOH-terminal domain of Eps15 or a mutant form lacking all the AP-2-binding sites was fused to the green fluorescent protein (GFP), and these constructs were transiently transfected in HeLa cells. Overexpression of the fusion protein containing the entire COOH-terminal domain of Eps15 strongly inhibited endocytosis of transferrin, whereas the fusion protein in which the AP-2-binding sites had been deleted had no effect. These results were confirmed in a cell-free assay that uses perforated A431 cells to follow the first steps of coated vesicle formation at the plasma membrane. Addition of Eps15-derived glutathione-S-transferase fusion proteins containing the AP-2-binding site in this assay inhibited not only constitutive endocytosis of transferrin but also ligand-induced endocytosis of epidermal growth factor. This inhibition could be ascribed to a competition between the fusion protein and endogenous Eps15 for AP-2 binding. Altogether, these results show that interaction of Eps15 with AP-2 is required for efficient receptor-mediated endocytosis and thus provide the first evidence that Eps15 is involved in the function of plasma membrane-coated pits.
Figures




References
-
- Beck KA, Keen JH. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4442–4447. - PubMed
-
- Benmerah A, Bègue B, Dautry-Varsat A, Cerf-Bensussan N. The ear of α-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J Biol Chem. 1996;271:12111–12116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

