Intracellular localization of phosphatidylinositide 3-kinase and insulin receptor substrate-1 in adipocytes: potential involvement of a membrane skeleton
- PMID: 9490733
- PMCID: PMC2132698
- DOI: 10.1083/jcb.140.5.1211
Intracellular localization of phosphatidylinositide 3-kinase and insulin receptor substrate-1 in adipocytes: potential involvement of a membrane skeleton
Abstract
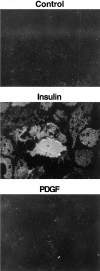
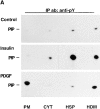
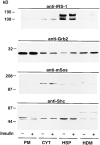
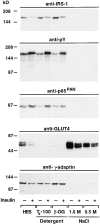
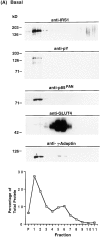
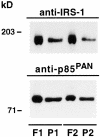
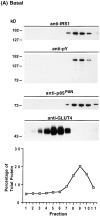
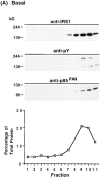
Phosphatidylinositide (PI) 3-kinase binds to tyrosyl-phosphorylated insulin receptor substrate-1 (IRS-1) in insulin-treated adipocytes, and this step plays a central role in the regulated movement of the glucose transporter, GLUT4, from intracellular vesicles to the cell surface. PDGF, which also activates PI 3-kinase in adipocytes, has no significant effect on GLUT4 trafficking in these cells. We propose that this specificity may be mediated by differential localization of PI 3-kinase in response to insulin versus PDGF activation. Using subcellular fractionation in 3T3-L1 adipocytes, we show that insulin- and PDGF-stimulated PI 3-kinase activities are located in an intracellular high speed pellet (HSP) and in the plasma membrane (PM), respectively. The HSP is also enriched in IRS-1, insulin-stimulated tyrosyl-phosphorylated IRS-1 and intracellular GLUT4-containing vesicles. Using sucrose density gradient sedimentation, we have been able to segregate the HSP into two separate subfractions: one enriched in IRS-1, tyrosyl-phosphorylated IRS-1, PI 3-kinase as well as cytoskeletal elements, and another enriched in membranes, including intracellular GLUT4 vesicles. Treatment of the HSP with nonionic detergent, liberates all membrane constituents, whereas IRS-1 and PI 3-kinase remain insoluble. Conversely, at high ionic strength, membranes remain intact, whereas IRS-1 and PI 3-kinase become freely soluble. We further show that this IRS-1-PI 3-kinase complex exists in CHO cells overexpressing IRS-1 and, in these cells, the cytosolic pool of IRS-1 and PI 3-kinase is released subsequent to permeabilization with Streptolysin-O, whereas the particulate fraction of these proteins is retained. These data suggest that IRS-1, PI 3-kinase, as well as other signaling intermediates, may form preassembled complexes that may be associated with the actin cytoskeleton. This complex must be in close apposition to the cell surface, enabling access to the insulin receptor and presumably other signaling molecules that somehow confer the absolute specificity of insulin signaling in these cells.
Figures











References
-
- Baltensperger K, Kozma LM, Cherniack AD, Klarlund JK, Chawla A, Banerjee U, Czech MP. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993;260:1950–1952. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
-
- Cambier JC, Campbell KS. Membrane immunoglobulin and its accomplices: new lessons from an old receptor. FASEB (Fed Am Soc Exp Biol) J. 1992;6:3207–3217. - PubMed
-
- Conricode KM. Involvement of phosphatidylinositol 3-kinase in stimulation of glucose transport by growth factors in 3T3-L1 adipocytes. Biochem Mol Biol Int. 1995;36:835–843. - PubMed
-
- Cormont M, Van Obbergen E, Zerial M, Le Marchand-Brustel Y. Insulin induces a change in rab5 subcellular localization in adipocytes independently of phosphatidylinositol 3-kinase activation. Endocrinology. 1996;137:3408–3415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

