Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: corralling and tethering by the membrane skeleton
- PMID: 9490734
- PMCID: PMC2132701
- DOI: 10.1083/jcb.140.5.1227
Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: corralling and tethering by the membrane skeleton
Abstract
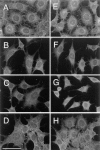
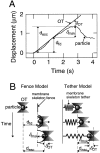
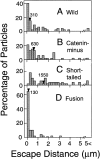
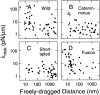
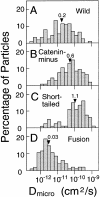
The translational movement of E-cadherin, a calcium-dependent cell-cell adhesion molecule in the plasma membrane in epithelial cells, and the mechanism of its regulation were studied using single particle tracking (SPT) and optical tweezers (OT). The wild type (Wild) and three types of artificial cytoplasmic mutants of E-cadherin were expressed in L-cells, and their movements were compared. Two mutants were E-cadherins that had deletions in the COOH terminus and lost the catenin-binding site(s) in the COOH terminus, with remaining 116 and 21 amino acids in the cytoplasmic domain (versus 152 amino acids for Wild); these are called Catenin-minus and Short-tailed in this paper, respectively. The third mutant, called Fusion, is a fusion protein between E-cadherin without the catenin-binding site and alpha-catenin without its NH2-terminal half. These cadherins were labeled with 40-nm phi colloidal gold or 210-nm phi latex particles via a monoclonal antibody to the extracellular domain of E-cadherin for SPT or OT experiments, respectively. E-cadherin on the dorsal cell surface (outside the cell-cell contact region) was investigated. Catenin-minus and Short-tailed could be dragged an average of 1.1 and 1.8 micron by OT (trapping force of 0.8 pN), and exhibited average microscopic diffusion coefficients (Dmicro) of 1.2 x 10(-10) and 2.1 x 10(-10) cm2/s, respectively. Approximately 40% of Wild, Catenin-minus, and Short-tailed exhibited confined-type diffusion. The confinement area was 0.13 micron2 for Wild and Catenin-minus, while that for Short-tailed was greater by a factor of four. In contrast, Fusion could be dragged an average of only 140 nm by OT. Average Dmicro for Fusion measured by SPT was small (0.2 x 10(-10) cm2/s). These results suggest that Fusion was bound to the cytoskeleton. Wild consists of two populations; about half behaves like Catenin- minus, and the other half behaves like Fusion. It is concluded that the movements of the wild-type E-cadherin in the plasma membrane are regulated via the cytoplasmic domain by (a) tethering to actin filaments through catenin(s) (like Fusion) and (b) a corralling effect of the network of the membrane skeleton (like Catenin-minus). The effective spring constants of the membrane skeleton that contribute to the tethering and corralling effects as measured by the dragging experiments were 30 and 5 pN/micron, respectively, indicating a difference in the skeletal structures that produce these two effects.
Figures







References
-
- Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Observation of a single-beam gradient force optical trap for dielectric particles. Optics Lett. 1986;11:288–290. - PubMed
-
- Ashkin A, Dziedzic JM, Yamane T. Optical trapping and manipulation of single cells using infrared-laser beams. Nature. 1987;330:769–771. - PubMed
-
- Balsamo J, Lilien J. N-cadherin is stably associated with and is an acceptor for a cell surface N-acetylgalactosaminylphosphotransferase. J Biol Chem. 1990;265:2923–2928. - PubMed
-
- Berg, H.C. 1983. Random Walks in Biology. Princeton University Press, Princeton, New Jersey.

