Transport, Compartmentation, and Metabolism of Homoserine in Higher Plant Cells. Carbon-13- and phosphorus-31-nuclear magnetic resonance studies Carbon-13- and Phosphorus-31-Nuclear Magnetic Resonance Studies
- PMID: 9490758
- PMCID: PMC35112
- DOI: 10.1104/pp.116.2.547
Transport, Compartmentation, and Metabolism of Homoserine in Higher Plant Cells. Carbon-13- and phosphorus-31-nuclear magnetic resonance studies Carbon-13- and Phosphorus-31-Nuclear Magnetic Resonance Studies
Abstract
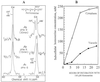
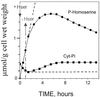
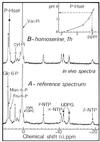
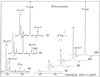
The transport, compartmentation, and metabolism of homoserine was characterized in two strains of meristematic higher plant cells, the dicotyledonous sycamore (Acer pseudoplatanus) and the monocotyledonous weed Echinochloa colonum. Homoserine is an intermediate in the synthesis of the aspartate-derived amino acids methionine, threonine (Thr), and isoleucine. Using 13C-nuclear magnetic resonance, we showed that homoserine actively entered the cells via a high-affinity proton-symport carrier (Km approximately 50-60 mum) at the maximum rate of 8 +/- 0.5 mumol h-1 g-1 cell wet weight, and in competition with serine or Thr. We could visualize the compartmentation of homoserine, and observed that it accumulated at a concentration 4 to 5 times higher in the cytoplasm than in the large vacuolar compartment. 31P-nuclear magnetic resonance permitted us to analyze the phosphorylation of homoserine. When sycamore cells were incubated with 100 mum homoserine, phosphohomoserine steadily accumulated in the cytoplasmic compartment over 24 h at the constant rate of 0.7 mumol h-1 g-1 cell wet weight, indicating that homoserine kinase was not inhibited in vivo by its product, phosphohomoserine. The rate of metabolism of phosphohomoserine was much lower (0.06 mumol h-1 g-1 cell wet weight) and essentially sustained Thr accumulation. Similarly, homoserine was actively incorporated by E. colonum cells. However, in contrast to what was seen in sycamore cells, large accumulations of Thr were observed, whereas the intracellular concentration of homoserine remained low, and phosphohomoserine did not accumulate. These differences with sycamore cells were attributed to the presence of a higher Thr synthase activity in this strain of monocot cells.
Figures



References
-
- Aubert S, Alban C, Bligny R, Douce R. Induction of β-methylcrotonyl-coenzyme A carboxylase in higher plant cells during carbohydrate starvation: evidence for a role of MCCase in leucine catabolism. FEBS Lett. 1996a;383:175–180. - PubMed
-
- Aubert S, Bligny R, Day DA, Whelan J, Douce R. Induction of alternative oxidase synthesis by herbicides inhibiting branched-chain amino acid synthesis. Plant J. 1997;11:649–657.
-
- Aubert S, Bligny R, Douce R (1996b) NMR studies of metabolism in cell suspensions and tissue cultures. In Y Shachar-Hill, P Pfeffer, eds, Nuclear Magnetic Resonance in Plant Physiology. American Society of Plant Physiologists, Rockville, MD, pp 109–154
-
- Aubert S, Gout E, Bligny R, Mazars-Marty D, Barrieu F, Alabouvette J, Marty F, Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells submitted to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996c;133:1251–1263. - PMC - PubMed
-
- Bligny R, Gardestrom P, Roby C, Douce R. 31P NMR studies of spinach leaves and their chloroplasts. J Biol Chem. 1990;265:1319–1326. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

