Oxidative Burst and Hypoosmotic Stress in Tobacco Cell Suspensions
- PMID: 9490766
- PMCID: PMC35124
- DOI: 10.1104/pp.116.2.659
Oxidative Burst and Hypoosmotic Stress in Tobacco Cell Suspensions
Abstract
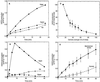
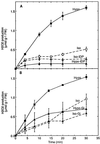
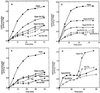
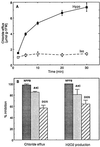
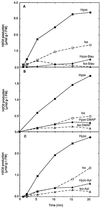
Oxidative burst constitutes an early response in plant defense reactions toward pathogens, but active oxygen production may also be induced by other stimuli. The oxidative response of suspension-cultured tobacco (Nicotiana tabacum cv Xanthi) cells to hypoosmotic and mechanical stresses was characterized. The oxidase involved in the hypoosmotic stress response showed similarities by its NADPH dependence and its inhibition by iodonium diphenyl with the neutrophil NADPH oxidase. Activation of the oxidative response by hypoosmotic stress needed protein phosphorylation and anion effluxes, as well as opening of Ca2+ channels. Inhibition of the oxidative response impaired Cl- efflux, K+ efflux, and extracellular alkalinization, suggesting that the oxidative burst may play a role in ionic flux regulation. Active oxygen species also induced the cross-linking of a cell wall protein, homologous to a soybean (Glycine max L.) extensin, that may act as part of cell volume and turgor regulation through modification of the physical properties of the cell wall.
Figures


References
-
- Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99.
-
- Bottin A, Véronési C, Pontier D, Esquerré-Tugayé MT, Blein JP, Rusterucci C, Ricci P. Differential responses of tobacco cells to elicitors from two Phytophtora species. Plant Physiol Biochem. 1994;32:373–378.
-
- Braam J, Davis RW. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. - PubMed
-
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

