Calcium-Dependent Protein Phosphorylation May Mediate the Gibberellic Acid Response in Barley Aleurone
- PMID: 9490770
- PMCID: PMC35136
- DOI: 10.1104/pp.116.2.765
Calcium-Dependent Protein Phosphorylation May Mediate the Gibberellic Acid Response in Barley Aleurone
Abstract
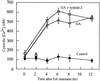
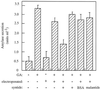
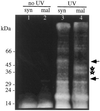
Peptide substrates of well-defined protein kinases were microinjected into aleurone protoplasts of barley (Hordeum vulgare L. cv Himalaya) to inhibit, and therefore identify, protein kinase-regulated events in the transduction of the gibberellin (GA) and abscisic acid signals. Syntide-2, a substrate designed for Ca2+- and calmodulin (CaM)-dependent kinases, selectively inhibited the GA response, leaving constitutive and abscisic acid-regulated events unaffected. Microinjection of syntide did not affect the GA-induced increase in cytosolic [Ca2+], suggesting that it inhibited GA action downstream of the Ca2+ signal. When photoaffinity-labeled syntide-2 was electroporated into protoplasts and cross-linked to interacting proteins in situ, it selectively labeled proteins of approximately 30 and 55 kD. A 54-kD, soluble syntide-2 phosphorylating protein kinase was detected in aleurone cells. This kinase was activated by Ca2+ and was CaM independent, but was inhibited by the CaM antagonist N-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide (250 mum), suggesting that it was a CaM-domain protein kinase-like activity. These results suggest that syntide-2 inhibits the GA response of the aleurone via an interaction with this kinase, implicating the 54-kD kinase as a Ca2+-dependent regulator of the GA response in these cells.
Figures




Similar articles
-
Signal Transduction in Barley Aleurone Protoplasts Is Calcium Dependent and Independent.Plant Cell. 1996 Dec;8(12):2193-2209. doi: 10.1105/tpc.8.12.2193. Plant Cell. 1996. PMID: 12239376 Free PMC article.
-
Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts.Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3591-5. doi: 10.1073/pnas.89.8.3591. Proc Natl Acad Sci U S A. 1992. PMID: 1533046 Free PMC article.
-
Perception of Gibberellin and Abscisic Acid at the External Face of the Plasma Membrane of Barley (Hordeum vulgare L.) Aleurone Protoplasts.Plant Physiol. 1994 Apr;104(4):1185-1192. doi: 10.1104/pp.104.4.1185. Plant Physiol. 1994. PMID: 12232156 Free PMC article.
-
Gibberellin and abscisic acid signalling in aleurone.Trends Plant Sci. 2000 Mar;5(3):102-10. doi: 10.1016/s1360-1385(00)01571-5. Trends Plant Sci. 2000. PMID: 10707075 Review.
-
Neuronal Ca2+/calmodulin-dependent protein kinases.Annu Rev Biochem. 1992;61:559-601. doi: 10.1146/annurev.bi.61.070192.003015. Annu Rev Biochem. 1992. PMID: 1323238 Review.
Cited by
-
Expression of a gibberellin-induced leucine-rich repeat receptor-like protein kinase in deepwater rice and its interaction with kinase-associated protein phosphatase.Plant Physiol. 1999 Jun;120(2):559-70. doi: 10.1104/pp.120.2.559. Plant Physiol. 1999. PMID: 10364408 Free PMC article.
-
An Arabidopsis calcium-dependent protein kinase is associated with the endoplasmic reticulum.Plant Physiol. 2002 Mar;128(3):1008-21. doi: 10.1104/pp.010770. Plant Physiol. 2002. PMID: 11891256 Free PMC article.
-
Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity.Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2697-702. doi: 10.1073/pnas.95.5.2697. Proc Natl Acad Sci U S A. 1998. PMID: 9482950 Free PMC article.
-
Molecular and biochemical characterization of the Capsicum annuum calcium-dependent protein kinase 3 (CaCDPK3) gene induced by abiotic and biotic stresses.Planta. 2004 Dec;220(2):286-95. doi: 10.1007/s00425-004-1372-9. Epub 2004 Sep 24. Planta. 2004. PMID: 15449060
-
Identification of phosphoproteins regulated by gibberellin in rice leaf sheath.Plant Mol Biol. 2005 May;58(1):27-40. doi: 10.1007/s11103-005-4013-1. Plant Mol Biol. 2005. PMID: 16028114
References
-
- Aromatorio DK, Parker J, Brown WE. High-resolution analytical and preparative peptide mapping by a combination of ion-exchange chromatography and thin-layer chromatography. Methods Enzymol. 1983;91:384–391. - PubMed
-
- Ashizawa K, Wishart GJ, Hashimoto K, Tsuzuki Y. Dephosphorylation of a 30 kD protein kinase of fowl spermatozoa by the addition of myosin light chain substrate peptide inhibits the flagellar motility. Biochem Biophys Res Commun. 1995;215:706–712. - PubMed
-
- Banks PR, Paquette DM. Comparison of three common reactive fluorescent probes used for conjugation to biomolecules by capillary zone electrophoresis. Bioconjugate Chem. 1995;6:447–458. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

