Identification of a Role for an Azide-Sensitive Factor in the Thylakoid Transport of the 17-Kilodalton Subunit of the Photosynthetic Oxygen-Evolving Complex
- PMID: 9490772
- PMCID: PMC35140
- DOI: 10.1104/pp.116.2.805
Identification of a Role for an Azide-Sensitive Factor in the Thylakoid Transport of the 17-Kilodalton Subunit of the Photosynthetic Oxygen-Evolving Complex
Abstract
We have examined the transport of the precursor of the 17-kD subunit of the photosynthetic O2-evolving complex (OE17) in intact chloroplasts in the presence of inhibitors that block two protein-translocation pathways in the thylakoid membrane. This precursor uses the transmembrane pH gradient-dependent pathway into the thylakoid lumen, and its transport across the thylakoid membrane is thought to be independent of ATP and the chloroplast SecA homolog, cpSecA. We unexpectedly found that azide, widely considered to be an inhibitor of cpSecA, had a profound effect on the targeting of the photosynthetic OE17 to the thylakoid lumen. By itself, azide caused a significant fraction of mature OE17 to accumulate in the stroma of intact chloroplasts. When added in conjunction with the protonophore nigericin, azide caused the maturation of a fraction of the stromal intermediate form of OE17, and this mature protein was found only in the stroma. Our data suggest that OE17 may use the sec-dependent pathway, especially when the transmembrane pH gradient-dependent pathway is inhibited. Under certain conditions, OE17 may be inserted across the thylakoid membrane far enough to allow removal of the transit peptide, but then may slip back out of the translocation machinery into the stromal compartment.
Figures
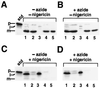


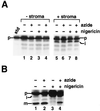
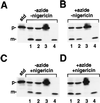
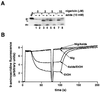
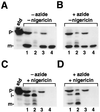

References
-
- Berghofer J, Karnauchov I, Herrmann RG, Klosgen RB. Isolation and characterization of a cDNA encoding the SecA protein from spinach chloroplasts: evidence for azide resistance of sec-dependent protein translocation across thylakoid membranes in spinach. J Biol Chem. 1995;270:18341–18346. - PubMed
-
- Brock IW, Mills JD, Robinson D, Robinson C. The ΔpH-driven, ATP-independent protein translocation mechanism in the chloroplast thylakoid membrane. J Biol Chem. 1995;270:1657–1662. - PubMed
-
- Clausmeyer S, Klosgen RB, Herrmann RG. Protein import into chloroplasts: the hydrophilic lumenal proteins exhibit unexpected import and sorting specificities in spite of structurally conserved transit peptides. J Biol Chem. 1993;268:13869–13876. - PubMed
-
- Cline K, Ettinger WF, Theg SM. Protein-specific energy requirements for protein transport across or into thylakoid membranes: two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992;267:2688–2696. - PubMed
-
- Cline K, Henry R. Import and routing of nucleus-encoded chloroplast proteins. Annu Rev Cell Biol. 1996;12:1–26. - PubMed
LinkOut - more resources
Full Text Sources

