The human Na+-glucose cotransporter is a molecular water pump
- PMID: 9490810
- PMCID: PMC2230848
- DOI: 10.1111/j.1469-7793.1998.015br.x
The human Na+-glucose cotransporter is a molecular water pump
Abstract
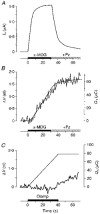
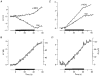
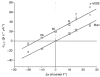
1. The human Na+-glucose cotransporter (hSGLT1) was expressed in Xenopus laevis oocytes. The transport activity, given by the Na+ current, was monitored as a clamp current and the concomitant flux of water followed optically as the change in oocyte volume. 2. When glucose was added to the bathing solution there was an abrupt increase in clamp current and an immediate swelling of the oocyte. The transmembrane transport of two Na+ ions and one sugar molecule was coupled, within the protein itself, to the influx of 210 water molecules. 3. This stoichiometry was constant and independent of the external parameters: Na+ concentrations, sugar concentrations, transmembrane voltages, temperature and osmotic gradients. 4. The cotransport of water occurred in the presence of adverse osmotic gradients. In accordance with the Gibbs equation, energy was transferred within the protein from the downhill fluxes of Na+ and sugar to the uphill transport of water, indicative of secondary active transport of water. 5. Unstirred layer effects were ruled out on the basis of experiments on oocytes treated with gramicidin or other ionophores. Na+ currents maintained by ionophores did not lead to any initial water movements. 6. The finding of a molecular water pump allows for new models of cellular water transport which include coupling between ion and water fluxes at the protein level; the hSGLT1 could account for almost half the daily reuptake of water from the small intestine.
Figures
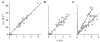
References
-
- Colombo MF, Rau DC, Parsegian VA. Protein solvation in allosteric regulation: A water effect on hemoglobin. Science. 1992;256:655–659. - PubMed
-
- Gutiérrez AM, Gonzalez E, Echevarria M, Hernandez CS, Whittembury G. The proximal straight tubule (PST) basolateral cell membrane water channel: selectivity characteristics. Journal of Membrane Biology. 1995;143:189–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

