Properties of human glycine receptors containing the hyperekplexia mutation alpha1(K276E), expressed in Xenopus oocytes
- PMID: 9490812
- PMCID: PMC2230779
- DOI: 10.1111/j.1469-7793.1998.025bu.x
Properties of human glycine receptors containing the hyperekplexia mutation alpha1(K276E), expressed in Xenopus oocytes
Abstract
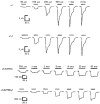
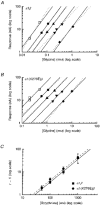
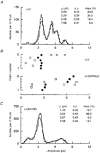
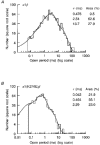
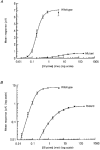
1. Inherited defects in human glycine receptors give rise to hyperekplexia (startle disease). We expressed human glycine receptors in Xenopus oocytes, in order to examine the pharmacological and single-channel properties of receptors that contain a mutation, alpha1(K276E), associated with an atypical form of hyperekplexia. 2. Equilibrium concentration-response curves showed that recombinant human alpha1(K276E)beta receptors had a 29-fold lower glycine sensitivity than wild-type alpha1beta receptors, and a greatly reduced Hill coefficient. The maximum response to glycine also appeared much reduced, whereas the equilibrium constant for the glycine receptor antagonist strychnine was unchanged. 3. Both wild-type and mutant channels opened to multiple conductance levels with similar main conductance levels (33 pS) and weighted mean conductances (41.5 versus 49.8 pS, respectively). 4. Channel openings were shorter for the alpha1(K276E)beta mutant than for the wild-type alpha1beta, with mean overall apparent open times of 0.82 and 6.85 ms, respectively. 5. The main effect of the alpha1(K276E) mutation is to impair the opening of the channel rather than the binding of glycine. This is shown by the results of fitting glycine dose-response curves with particular postulated mechanisms, the shorter open times of mutant channels, the properties of single-channel bursts, and the lack of an effect of the mutation on the strychnine-binding site.
Figures


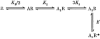
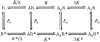
Similar articles
-
The α1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors.J Neurosci. 2012 Jan 25;32(4):1336-52. doi: 10.1523/JNEUROSCI.4346-11.2012. J Neurosci. 2012. PMID: 22279218 Free PMC article.
-
Decreased agonist affinity and chloride conductance of mutant glycine receptors associated with human hereditary hyperekplexia.EMBO J. 1994 Sep 15;13(18):4223-8. doi: 10.1002/j.1460-2075.1994.tb06742.x. EMBO J. 1994. PMID: 7925268 Free PMC article.
-
The startle disease mutation Q266H, in the second transmembrane domain of the human glycine receptor, impairs channel gating.Mol Pharmacol. 1999 Feb;55(2):386-95. doi: 10.1124/mol.55.2.386. Mol Pharmacol. 1999. PMID: 9927632
-
The genetics of hyperekplexia: more than startle!Trends Genet. 2008 Sep;24(9):439-47. doi: 10.1016/j.tig.2008.06.005. Epub 2008 Aug 15. Trends Genet. 2008. PMID: 18707791 Review.
-
Hyperekplexia: a treatable neurogenetic disease.Brain Dev. 2002 Oct;24(7):669-74. doi: 10.1016/s0387-7604(02)00095-5. Brain Dev. 2002. PMID: 12427512 Review.
Cited by
-
Role of aspartate 298 in mouse 5-HT3A receptor gating and modulation by extracellular Ca2+.J Physiol. 2005 Oct 15;568(Pt 2):381-96. doi: 10.1113/jphysiol.2005.092866. Epub 2005 Aug 11. J Physiol. 2005. PMID: 16096341 Free PMC article.
-
The activation mechanism of alpha1 homomeric glycine receptors.J Neurosci. 2004 Jan 28;24(4):895-906. doi: 10.1523/JNEUROSCI.4420-03.2004. J Neurosci. 2004. PMID: 14749434 Free PMC article.
-
Exploring the Conformational Impact of Glycine Receptor TM1-2 Mutations Through Coarse-Grained Analysis and Atomistic Simulations.Front Mol Biosci. 2022 Jun 28;9:890851. doi: 10.3389/fmolb.2022.890851. eCollection 2022. Front Mol Biosci. 2022. PMID: 35836931 Free PMC article.
-
Inhibitory glycine receptors: an update.J Biol Chem. 2012 Nov 23;287(48):40216-23. doi: 10.1074/jbc.R112.408229. Epub 2012 Oct 4. J Biol Chem. 2012. PMID: 23038260 Free PMC article. Review.
-
NMR structures of the second transmembrane domain of the human glycine receptor alpha(1) subunit: model of pore architecture and channel gating.Biophys J. 2002 Jul;83(1):252-62. doi: 10.1016/S0006-3495(02)75166-7. Biophys J. 2002. PMID: 12080117 Free PMC article.
References
-
- Baxter P, Connolly S, Curtis A, Spencer V, Ravindra Nath C, Burn J, Gardner-Medwin D. Co-dominant inheritance of hyperekplexia and spastic paraparesis. Developmental Medicine and Child Neurology. 1996;38:739–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

