Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn
- PMID: 9490821
- PMCID: PMC2230847
- DOI: 10.1111/j.1469-7793.1998.083br.x
Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn
Abstract
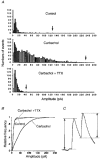
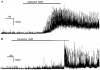
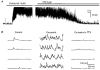
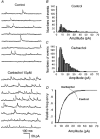
1. Blind patch clamp recordings were made from substantia gelatinosa (SG) neurones in the adult rat spinal cord slice to study the mechanisms of cholinergic modulation of GABAergic inhibition. 2. In the majority of SG neurones tested, carbachol (10 microM) increased the frequency (677 % of control) of spontaneous GABAergic inhibitory postsynaptic currents (IPSCs). A portion of these events appeared to result from the generation of spikes by GABAergic interneurones, since large amplitude IPSCs were eliminated by tetrodotoxin (1 microM). 3. The effect of carbachol on spontaneous IPSCs was mimicked by neostigmine, suggesting that GABAergic interneurones are under tonic regulation by cholinergic systems. 4. The frequency of GABAergic miniature IPSCs in the presence of tetrodotoxin (1 microM) was also increased by carbachol without affecting amplitude distribution, indicating that acetylcholine facilitates quantal release of GABA through presynaptic mechanisms. 5. Neither the M1 receptor agonist McN-A-343 (10-300 microM) nor the M2 receptor agonist, arecaidine (10-100 microM), mimicked the effects of carbachol. All effects of carbachol and neostigmine were antagonized by atropine (1 muM), while pirenzepine (100 nM), methoctramine (1 microM) and hexahydrosiladifenidol hydrochloride, p-fluoro-analog (100 nM) had no effect. 6. Focal stimulation of deep dorsal horn, but not dorsolateral funiculus, evoked a similar increase in IPSC frequency to that evoked by carbachol and neostigmine. The stimulation-induced facilitation of GABAergic transmission lasted for 2-3 min post stimulation, and the effect was antagonized by atropine (100 nM). 7. Our observations suggest that GABAergic interneurones possess muscarinic receptors on both axon terminals and somatodendritic sites, that the activation of these receptors increases the excitability of inhibitory interneurones and enhances GABA release in SG and that the GABAergic inhibitory system is further controlled by cholinergic neurones located in the deep dorsal horn. Those effects may be responsible for the antinociceptive action produced by the intrathecal administration of muscarinic agonists and acetylcholinesterase inhibitors.
Figures




Similar articles
-
Postsynaptic integration of cholinergic and dopaminergic signals on medium-sized GABAergic projection neurons in the neostriatum.Brain Res Bull. 1998 Apr;45(6):607-13. doi: 10.1016/s0361-9230(97)00460-7. Brain Res Bull. 1998. PMID: 9566505
-
Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons.Anesthesiology. 2000 Feb;92(2):485-92. doi: 10.1097/00000542-200002000-00031. Anesthesiology. 2000. PMID: 10691236
-
Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice.J Physiol. 1992 May;450:127-42. doi: 10.1113/jphysiol.1992.sp019119. J Physiol. 1992. PMID: 1359121 Free PMC article.
-
Muscarinic-mediated analgesia.Life Sci. 1999;64(6-7):549-54. doi: 10.1016/s0024-3205(98)00600-6. Life Sci. 1999. PMID: 10069522 Review.
-
Metabolic and neurophysiologic sequelae of brain injury: a cholinergic hypothesis.Cent Nerv Syst Trauma. 1986 Spring;3(2):163-73. doi: 10.1089/cns.1986.3.163. Cent Nerv Syst Trauma. 1986. PMID: 3533278 Review.
Cited by
-
The Role of Retinal Dysfunction in Myopia Development.Cell Mol Neurobiol. 2023 Jul;43(5):1905-1930. doi: 10.1007/s10571-022-01309-1. Epub 2022 Nov 24. Cell Mol Neurobiol. 2023. PMID: 36427109 Free PMC article. Review.
-
Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats.J Neurosci. 2006 Feb 8;26(6):1787-94. doi: 10.1523/JNEUROSCI.4856-05.2006. J Neurosci. 2006. PMID: 16467527 Free PMC article.
-
ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses.J Physiol. 2000 Apr 15;524 Pt 2(Pt 2):471-83. doi: 10.1111/j.1469-7793.2000.t01-1-00471.x. J Physiol. 2000. PMID: 10766927 Free PMC article.
-
Striatal input- and rate-dependent effects of muscarinic receptors on pallidal firing.ScientificWorldJournal. 2012;2012:547638. doi: 10.1100/2012/547638. Epub 2012 May 1. ScientificWorldJournal. 2012. PMID: 22654627 Free PMC article.
-
Activations of muscarinic M1 receptors in the anterior cingulate cortex contribute to the antinociceptive effect via GABAergic transmission.Mol Pain. 2017 Jan;13:1744806917692330. doi: 10.1177/1744806917692330. Mol Pain. 2017. PMID: 28326934 Free PMC article.
References
-
- Abram SE, O'Connor TC. Characteristics of the analgesic effects and drug interactions of intrathecal carbachol in rats. Anesthesiology. 1995;83:844–849. 10.1097/00000542-199510000-00025. - DOI - PubMed
-
- Benardo LS, Prince DA. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Research. 1982;249:315–331. - PubMed
-
- Borges LF, Iversen SD. Topography of choline acetyltransferase immunoreactive neurons and fibers in the rat spinal cord. Brain Research. 1986;362:140–148. - PubMed
-
- Bouaziz H, Tong C, Eisenach JC. Postoperative analgesia from intrathecal neostigmine in sheep. Anesthesia & Analgesia. 1995;80:1140–1144. - PubMed
-
- Brown AG. Organization in the Spinal Cord. Berlin: Springer-Verlag; 1981.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

