Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure
- PMID: 9490839
- PMCID: PMC2230857
- DOI: 10.1111/j.1469-7793.1998.199br.x
Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure
Abstract
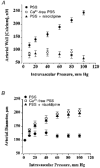
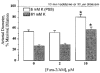
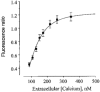
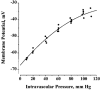
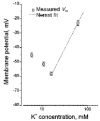
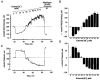
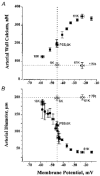
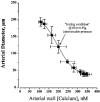
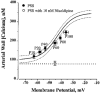
1. The regulation of intracellular [Ca2+] in the smooth muscle cells in the wall of small pressurized cerebral arteries (100-200 micron) of rat was studied using simultaneous digital fluorescence video imaging of arterial diameter and wall [Ca2+], combined with microelectrode measurements of arterial membrane potential. 2. Elevation of intravascular pressure (from 10 to 100 mmHg) caused a membrane depolarization from -63 +/- 1 to -36 +/- 2 mV, increased arterial wall [Ca2+] from 119 +/- 10 to 245 +/- 9 nM, and constricted the arteries from 208 +/- 10 micron (fully dilated, Ca2+ free) to 116 +/- 7 micron or by 45 % ('myogenic tone'). 3. Pressure-induced increases in arterial wall [Ca2+] and vasoconstriction were blocked by inhibitors of voltage-dependent Ca2+ channels (diltiazem and nisoldipine) or to the same extent by removal of external Ca2+. 4. At a steady pressure (i.e. under isobaric conditions at 60 mmHg), the membrane potential was stable at -45 +/- 1 mV, intracellular [Ca2+] was 190 +/- 10 nM, and arteries were constricted by 41 % (to 115 +/- 7 micron from 196 +/- 8 micron fully dilated). Under this condition of -45 +/- 5 mV at 60 mmHg, the voltage sensitivity of wall [Ca2+] and diameter were 7.5 nM mV-1 and 7.5 micron mV-1, respectively, resulting in a Ca2+ sensitivity of diameter of 1 mum nM-1. 5. Membrane potential depolarization from -58 to -23 mV caused pressurized arteries (to 60 mmHg) to constrict over their entire working range, i.e. from maximally dilated to constricted. This depolarization was associated with an elevation of arterial wall [Ca2+] from 124 +/- 7 to 347 +/- 12 nM. These increases in arterial wall [Ca2+] and vasoconstriction were blocked by L-type voltage-dependent Ca2+ channel inhibitors. 6. The relationship between arterial wall [Ca2+] and membrane potential was not significantly different under isobaric (60 mmHg) and non-isobaric conditions (10-100 mmHg), suggesting that intravascular pressure regulates arterial wall [Ca2+] through changes in membrane potential. 7. The results are consistent with the idea that intravascular pressure causes membrane potential depolarization, which opens voltage-dependent Ca2+ channels, acting as 'voltage sensors', thus increasing Ca2+ entry and arterial wall [Ca2+], which leads to vasoconstriction.
Figures
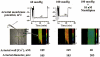

References
-
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. - PubMed
-
- Chen XL, Rembold CM. Phenylephrine contracts rat tail artery by one electromechanical and three pharmacomechanical mechanisms. American Journal of Physiology. 1995;268:H74–81. - PubMed
-
- D'Angelo G, Davis MJ, Meininger GA. Calcium and mechanotransduction of the myogenic response. American Journal of Physiology. 1997;273:H175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

