Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae
- PMID: 9490870
- PMCID: PMC2230716
- DOI: 10.1111/j.1469-7793.1998.431bw.x
Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae
Abstract
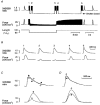
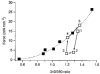
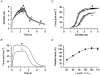
1. Changes in cytosolic [Ca2+] ([Ca2+]i) were measured in isolated rat trabeculae that had been micro-injected with fura-2 salt, in order to investigate the mechanism by which twitch force changes following an alteration of muscle length. 2. A step increase in length of the muscle produced a rapid potentiation of twitch force but not of the Ca2+ transient. The rapid rise of force was unaffected by inhibiting the sarcoplasmic reticulum (SR) with ryanodine and cyclopiazonic acid. 3. The force-[Ca2+]i relationship of the myofibrils in situ, determined from twitches and tetanic contractions in SR-inhibited muscles, showed that the rapid rise of force was due primarily to an increase in myofibrillar Ca2+ sensitivity, with a contribution from an increase in the maximum force production of the myofibrils. 4. After stretch of the muscle there was a further, slow increase of twitch force which was due entirely to a slow increase of the Ca2+ transient, since there was no change in the myofibrillar force-[Ca2+]i relationship. SR inhibition slowed down, but did not alter the magnitude of, the slow force response. 5. During the slow rise of force there was no slow increase of diastolic [Ca2+]i, whether or not the SR was inhibited. The same was true in unstimulated muscles. 6. We conclude that the rapid increase in twitch force after muscle stretch is due to the length-dependent properties of the myofibrils. The slow force increase is not explained by length dependence of the myofibrils or the SR, or by a rise in diastolic [Ca2+]i. Evidence from tetani suggests the slow force responses result from increased Ca2+ loading of the cell during the action potential.
Figures
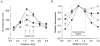


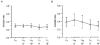

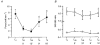

References
-
- Allen DG. On the relationship between action potential duration and tension in cat papillary muscle. Cardiovascular Research. 1977;11:210–218. - PubMed
-
- Allen DG, Kentish JC. The cellular basis of the length-tension relationship in cardiac muscle. Journal of Molecular and Cellular Cardiology. 1985;17:821–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

