A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria
- PMID: 9491824
- PMCID: PMC1873351
- DOI: 10.1046/j.1365-2125.1998.00655.x
A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria
Abstract
Aims: To obtain comprehensive pharmacokinetic and pharmacodynamic data for artesunate (ARTS) and its active metabolite dihydroartemisinin (DHA) following i.v. and oral administration of ARTS to patients with acute, uncomplicated falciparum malaria.
Methods: Twenty-six Vietnamese patients with falciparum malaria were randomized to receive either i.v. ARTS (120 mg; group 1) or oral ARTS (100 mg; group 2), with the alternative preparation given 8 h later in an open crossover design. Mefloquine (750 mg) was administered at 24 h. Plasma concentrations of ARTS and DHA were determined by h.p.l.c. assay. Pharmacokinetic parameters were calculated by non-compartmental methods. The time to 50% parasite clearance (PCT50) was calculated by linear interpolation of parasite density determinations. Linear least squares and multiple linear regression analyses were used to evaluate pharmacokinetic-pharmacodynamic relationships.
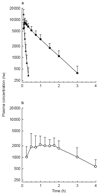
Results: Following i.v. bolus, ARTS had a peak concentration of 29.5 microM (11 mg l[-1]), elimination t1/2 = 2.7 min, CL = 2.33 l h(-1) kg(-1) and V = 0.14 l kg(-1). The Cmax for DHA was 9.3 microM (2.64 mg l[-1]), t1/2 = 40 min, CL =0.75 l h(-1) kg(-1) and V = 0.76 l kg(-1). Following oral ARTS, relative bioavailability of DHA was 82%, Cmax was 2.6 microM (0.74 mg l[-1]), t1/2 = 39 min, and MAT = 67 min. Overall, the PCT50 and fever clearance time (FCT) were 6.5 h and 24 h, respectively. There was no correlation between PCT50 or FCT and AUC, Cmax or MRT for DHA.
Conclusions: Despite rapid clearance of ARTS and DHA in patients with uncomplicated falciparum malaria, prompt parasite and fever clearance were achieved. High relative bioavailability of DHA following oral ARTS administration, and clinical outcomes comparable with those after i.v. ARTS, support the use of the oral formulation in the primary care setting.
Figures

References
-
- Price RN, Nosten F, Luxemburger C, et al. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. - PubMed
-
- Hien TT, White NJ. Qinghaosu. Lancet. 1993;341:603–608. - PubMed
-
- de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. - PubMed
-
- White NJ. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans R Soc Trop Med Hyg. 1994;88(Suppl 1):S41–S43. - PubMed
-
- Barradell LB, Fitton A. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

