The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes
- PMID: 9499054
- PMCID: PMC109493
- DOI: 10.1128/JVI.72.3.2002-2009.1998
The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes
Erratum in
- J Virol 1998 Sep;72(9):7707
Abstract
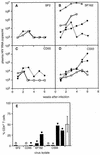
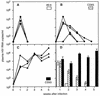
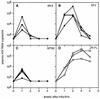
Most individuals infected with human immunodeficiency virus type 1 (HIV-1) initially harbor macrophage-tropic, non-syncytium-inducing (M-tropic, NSI) viruses that may evolve into T-cell-tropic, syncytium-inducing viruses (T-tropic, SI) after several years. The reasons for the more efficient transmission of M-tropic, NSI viruses and the slow evolution ofT-tropic, SI viruses remain unclear, although they may be linked to expression of appropriate chemokine coreceptors for virus entry. We have examined plasma viral RNA levels and the extent of CD4+ T-cell depletion in SCID mice reconstituted with human peripheral blood leukocytes following infection with M-tropic, dual-tropic, or T-tropic HIV-1 isolates. The cell tropism was found to determine the course of viremia, with M-tropic viruses producing sustained high viral RNA levels and sparing some CD4+ T cells, dual-tropic viruses producing a transient and lower viral RNA spike and extremely rapid depletion of CD4+ T cells, and T-tropic viruses causing similarly lower viral RNA levels and rapid-intermediate rates of CD4+ T-cell depletion. A single amino acid change in the V3 region of gp120 was sufficient to cause one isolate to switch from M-tropic to dual-tropic and acquire the ability to rapidly deplete all CD4+ T cells.
Figures



References
-
- Aldrovandi G, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I, Zack J. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. - PubMed
-
- Alkhatib G, Combadiere C, Broder C, Feng Y, Kennedy P, Murphy P, Berger E. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Autran B, Carcelain G, Li T, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. - PubMed
-
- Berger E. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(A):S3–S16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

