In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted
- PMID: 9499056
- PMCID: PMC109495
- DOI: 10.1128/JVI.72.3.2022-2032.1998
In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted
Abstract
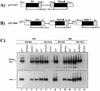
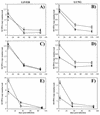
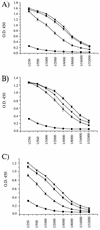
Isogenic, E3-deleted adenovirus vectors defective in E1, E1 and E2A, or E1 and E4 were generated in complementation cell lines expressing E1, E1 and E2A, or E1 and E4 and characterized in vitro and in vivo. In the absence of complementation, deletion of both E1 and E2A completely abolished expression of early and late viral genes, while deletion of E1 and E4 impaired expression of viral genes, although at a lower level than the E1/E2A deletion. The in vivo persistence of these three types of vectors was monitored in selected strains of mice with viral genomes devoid of transgenes to exclude any interference by immunogenic transgene-encoded products. Our studies showed no significant differences among the vectors in the short-term maintenance and long-term (4-month) persistence of viral DNA in liver and lung cells of immunocompetent and immunodeficient mice. Furthermore, all vectors induced similar antibody responses and comparable levels of adenovirus-specific cytotoxic T lymphocytes. These results suggest that in the absence of transgenes, the progressive deletion of the adenovirus genome does not extend the in vivo persistence of the transduced cells and does not reduce the antivirus immune response. In addition, our data confirm that, in the absence of transgene expression, mouse cellular immunity to viral antigens plays a minor role in the progressive elimination of the virus genome.
Figures

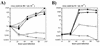



Similar articles
-
Generation of an adenovirus vector lacking E1, e2a, E3, and all of E4 except open reading frame 3.J Virol. 1999 Jul;73(7):6048-55. doi: 10.1128/JVI.73.7.6048-6055.1999. J Virol. 1999. PMID: 10364357 Free PMC article.
-
Generation and characterization of E1/E2a/E3/E4-deficient adenoviral vectors encoding human factor VIII.Mol Ther. 2001 Mar;3(3):329-36. doi: 10.1006/mthe.2001.0264. Mol Ther. 2001. PMID: 11273775
-
Development of cell lines capable of complementing E1, E4, and protein IX defective adenovirus type 5 mutants.Hum Gene Ther. 1995 Dec;6(12):1575-86. doi: 10.1089/hum.1995.6.12-1575. Hum Gene Ther. 1995. PMID: 8664382
-
[Development of a novel adenovirus vector exhibiting microRNA-mediated suppression of the leaky expression of adenovirus genes].Yakugaku Zasshi. 2012;132(12):1407-12. doi: 10.1248/yakushi.12-00235-5. Yakugaku Zasshi. 2012. PMID: 23208048 Review. Japanese.
-
Second-generation adenovirus vectors.Nat Med. 1996 Jun;2(6):714-6. doi: 10.1038/nm0696-714. Nat Med. 1996. PMID: 8640568 Review. No abstract available.
Cited by
-
Human adenovirus oncolytic properties and the inhibitory role of E4 orf4 and E4 orf6/7 on endogenously activated NF-κB.Biochem Biophys Rep. 2023 Dec 22;37:101616. doi: 10.1016/j.bbrep.2023.101616. eCollection 2024 Mar. Biochem Biophys Rep. 2023. PMID: 38205184 Free PMC article.
-
Fiberless recombinant adenoviruses: virus maturation and infectivity in the absence of fiber.J Virol. 1999 Feb;73(2):907-19. doi: 10.1128/JVI.73.2.907-919.1999. J Virol. 1999. PMID: 9882291 Free PMC article.
-
Recent advances in genetic modification of adenovirus vectors for cancer treatment.Cancer Sci. 2017 May;108(5):831-837. doi: 10.1111/cas.13228. Epub 2017 May 7. Cancer Sci. 2017. PMID: 28266780 Free PMC article. Review.
-
Adenoviral vector-based platforms for developing effective vaccines to combat respiratory viral infections.Clin Transl Immunology. 2021 Oct 12;10(10):e1345. doi: 10.1002/cti2.1345. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34667600 Free PMC article. Review.
-
NF-κB promotes leaky expression of adenovirus genes in a replication-incompetent adenovirus vector.Sci Rep. 2016 Jan 27;6:19922. doi: 10.1038/srep19922. Sci Rep. 2016. PMID: 26814140 Free PMC article.
References
-
- Barr D, Tubb J, Fergusson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. - PubMed
-
- Berkner K L. Development of adenovirus vectors for the expression of heterologous genes. BioTechniques. 1988;6:616–629. - PubMed
-
- Bosma G C, Custer M C, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature (London) 1983;301:527–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

