A peptide mimic of a protective epitope of respiratory syncytial virus selected from a combinatorial library induces virus-neutralizing antibodies and reduces viral load in vivo
- PMID: 9499058
- PMCID: PMC109497
- DOI: 10.1128/JVI.72.3.2040-2046.1998
A peptide mimic of a protective epitope of respiratory syncytial virus selected from a combinatorial library induces virus-neutralizing antibodies and reduces viral load in vivo
Abstract
Respiratory syncytial virus (RSV) is the most important cause of bronchiolitis and pneumonia in infants and young children worldwide. As yet, there is no effective vaccine against RSV infection, and previous attempts to develop a formalin-inactivated vaccine resulted in exacerbated disease in recipients subsequently exposed to the virus. In the work described here, a combinatorial solid-phase peptide library was screened with a protective monoclonal antibody (MAb 19) to identify peptide mimics (mimotopes) of a conserved and conformationally-determined epitope of RSV fusion (F) protein. Two sequences identified (S1 [HWYISKPQ] and S2 [HWYDAEVL]) reacted specifically with MAb 19 when they were presented as solid-phase peptides. Furthermore, after amino acid substitution analyses, three sequences derived from S1 (S1S [HWSISKPQ], S1K [KWYISKPQ], and S1P [HPYISKPQ]), presented as multiple antigen peptides (MAPs), also showed strong reactivity with MAb 19. The affinity constants of the binding of MAb 19, determined by surface plasmon resonance analyses, were 1.19 x 10(9) and 4.93 x 10(9) M(-1) for S1 and S1S, respectively. Immunization of BALB/c mice with these mimotopes, presented as MAPs, resulted in the induction of anti-peptide antibodies that inhibited the binding of MAb 19 to RSV and neutralized viral infection in vitro, with titers equivalent to those in sera from RSV-infected animals. Following RSV challenge of S1S mimotope-immunized mice, a 98.7% reduction in the titer of virus in the lungs was observed. Furthermore, there was a greatly reduced cell infiltration in the lungs of immunized mice compared to that in controls. These results indicate the potential of peptide mimotopes to protect against RSV infection without exacerbating pulmonary pathology.
Figures
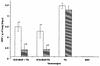
Similar articles
-
Peptide mimics of a conformationally constrained protective epitopes of respiratory syncytial virus fusion protein.Immunol Lett. 1997 Jun 1;57(1-3):15-7. doi: 10.1016/s0165-2478(97)00045-x. Immunol Lett. 1997. PMID: 9232419
-
Identification of multiple protective epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein.J Virol. 1999 Jul;73(7):5637-45. doi: 10.1128/JVI.73.7.5637-5645.1999. J Virol. 1999. PMID: 10364313 Free PMC article.
-
Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment.Virology. 1997 Apr 14;230(2):155-66. doi: 10.1006/viro.1997.8465. Virology. 1997. PMID: 9143271
-
Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years.Virus Res. 2011 Dec;162(1-2):80-99. doi: 10.1016/j.virusres.2011.09.020. Epub 2011 Sep 22. Virus Res. 2011. PMID: 21963675 Free PMC article. Review.
-
Respiratory syncytial virus infection and novel interventions.Nat Rev Microbiol. 2023 Nov;21(11):734-749. doi: 10.1038/s41579-023-00919-w. Epub 2023 Jul 12. Nat Rev Microbiol. 2023. PMID: 37438492 Review.
Cited by
-
A murine monoclonal antibody produced in transgenic plants with plant-specific glycans is not immunogenic in mice.Transgenic Res. 2000 Jun;9(3):187-94. doi: 10.1023/a:1008976219939. Transgenic Res. 2000. PMID: 11032367
-
Characterization of murine coronavirus neutralization epitopes with phage-displayed peptides.Virology. 2000 May 25;271(1):182-96. doi: 10.1006/viro.2000.0310. Virology. 2000. PMID: 10814583 Free PMC article.
-
Structure of a high-affinity "mimotope" peptide bound to HIV-1-neutralizing antibody b12 explains its inability to elicit gp120 cross-reactive antibodies.J Mol Biol. 2007 Jun 8;369(3):696-709. doi: 10.1016/j.jmb.2007.01.060. Epub 2007 Jan 27. J Mol Biol. 2007. PMID: 17445828 Free PMC article.
-
Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection.J Infect Dis. 2004 Sep 15;190(6):1119-26. doi: 10.1086/423286. Epub 2004 Aug 2. J Infect Dis. 2004. PMID: 15319862 Free PMC article.
-
Characterization of the conformational epitope of Guy's 13, a monoclonal antibody that prevents Streptococcus mutans colonization in humans.Infect Immun. 2003 Feb;71(2):754-65. doi: 10.1128/IAI.71.2.754-765.2003. Infect Immun. 2003. PMID: 12540555 Free PMC article.
References
-
- Arbiza J, Taylor G, Lopez J A, Furze J, Wyld S, Whyte P, Stott E J, Wertz G, Sullender W, Trudel M. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73:2225–2234. - PubMed
-
- Bourgeois C, Corvaisier C, Bour J B, Kohli E, Pothier P. Use of synthetic peptides to locate neutralizing antigenic domains on the fusion protein of respiratory syncytial virus. J Gen Virol. 1991;72:1051–1058. - PubMed
-
- Chanock R M, Parrott R H, Connors M, Collins P L, Murphy B R. Serious respiratory tract disease caused by respiratory syncytial virus: prospects for improved therapy and effective immunization. Pediatrics. 1992;90:137–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

