Efficient gap repair catalyzed in vitro by an intrinsic DNA polymerase activity of human immunodeficiency virus type 1 integrase
- PMID: 9499061
- PMCID: PMC109500
- DOI: 10.1128/JVI.72.3.2062-2071.1998
Efficient gap repair catalyzed in vitro by an intrinsic DNA polymerase activity of human immunodeficiency virus type 1 integrase
Abstract
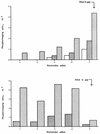
Cleavage and DNA joining reactions, carried out by human immunodeficiency virus type 1 (HIV-1) integrase, are necessary to effect the covalent insertion of HIV-1 DNA into the host genome. For the integration of HIV-1 DNA into the cellular genome to be completed, short gaps flanking the integrated proviral DNA must be repaired. It has been widely assumed that host cell DNA repair enzymes are involved. Here we report that HIV-1 integrase multimers possess an intrinsic DNA-dependent DNA polymerase activity. The activity was characterized by its dependence on Mg2+, resistance to N-ethylmaleimide, and inhibition by 3'-azido-2',3'-dideoxythymidine-5'-triphosphate, coumermycin A1, and pyridoxal 5'-phosphate. The enzyme efficiently utilized poly(dA)-oligo(dT) or self-annealing oligonucleotides as a template primer but displayed relatively low activity with gapped calf thymus DNA and no activity with poly(dA) or poly(rA)-oligo(dT). A monoclonal antibody binding specifically to an epitope comprised of amino acids 264 to 273 near the C terminus of HIV-1 integrase severely inhibited the DNA polymerase activity. A deletion of 50 amino acids at the C terminus of integrase drastically altered the gel filtration properties of the DNA polymerase, although the level of activity was unaffected by this mutation. The DNA polymerase efficiently extended a hairpin DNA primer up to 19 nucleotides on a T20 DNA template, although addition of the last nucleotide occurred infrequently or not at all. The ability of integrase to repair gaps in DNA was also investigated. We designed a series of gapped molecules containing a single-stranded region flanked by a duplex U5 viral arm on one side and by a duplex nonviral arm on the other side. Molecules varied structurally depending on the size of the gap (one, two, five, or seven nucleotides), their content of T's or C's in the single-stranded region, whether the CA dinucleotide in the viral arm had been replaced with a nonviral sequence, or whether they contained 5' AC dinucleotides as unpaired tails. The results indicated that the integrase DNA polymerase is specifically designed to repair gaps efficiently and completely, regardless of gap size, base composition, or structural features such as the internal CA dinucleotide or unpaired 5'-terminal AC dinucleotides. When the U5 arm of the gapped DNA substrate was removed, leaving a nongapped DNA template-primer, the integrase DNA polymerase failed to repair the last nucleotide in the DNA template effectively. A post-gap repair reaction did depend on the CA dinucleotide. This secondary reaction was highly regulated. Only two nucleotides beyond the gap were synthesized, and these were complementary to and dependent for their synthesis on the CA dinucleotide. We were also able to identify a specific requirement for the C terminus of integrase in the post-gap repair reaction. The results are consistent with a direct role for a heretofore unsuspected DNA polymerase function of HIV-1 integrase in the repair of short gaps flanking proviral DNA integration intermediates that arise during virus infection.
Figures
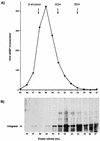
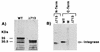

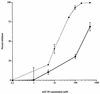
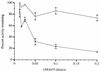
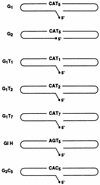

 , 20
ng.
, 20
ng.



References
-
- Andrake M D, Skalka A M. Multimerization determinants reside in both the catalytic core and C terminus of avian sarcoma virus integrase. J Biol Chem. 1995;270:29299–29306. - PubMed
-
- Andrake M D, Skalka A M. Retroviral integrase, putting the pieces together. J Biol Chem. 1996;271:19633–19636. - PubMed
-
- Barsov E V, Huber W E, Marcotrigiano J, Clark P K, Clark A D, Arnold E A, Hughes S H. Inhibition of human immunodeficiency virus type 1 integrase by the Fab fragment of a specific monoclonal antibody suggests that different multimerization states are required for different enzymatic functions. J Virol. 1996;70:4484–4494. - PMC - PubMed
-
- Burke C J, Sanyal G, Bruner M W, Ryan J A, LaFemina R L, Robbins H L, Zeft A S, Middaugh C R, Cordingley M G. Structural implications of spectroscopic characterization of a putative zinc finger peptide from HIV-1 integrase. J Biol Chem. 1992;267:9639–9644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

