A putative alpha-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly
- PMID: 9499062
- PMCID: PMC109501
- DOI: 10.1128/JVI.72.3.2072-2078.1998
A putative alpha-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly
Abstract
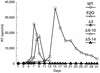
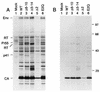
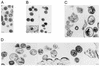
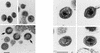
The capsid (CA) and nucleocapsid domains of the human immunodeficiency virus type 1 Gag polyprotein are separated by the p2 spacer peptide, which is essential for virus replication. Previous studies have revealed that p2 has an important role in virus morphogenesis. In this paper, we show that a crucial assembly determinant maps to the highly conserved N terminus of p2, which is predicted to form part of an alpha-helix that begins in CA. A mutational analysis indicates that the ability of the N terminus of p2 to adopt an alpha-helical structure is essential for its function during virus assembly. To prevent CA-p2 processing, it was necessary to mutate both the CA-p2 cleavage site and an internal cleavage site within p2. Virions produced by the double mutant lacked a conical core shell and instead contained a thin electron-dense shell about 10 nm underneath the virion membrane. These results suggest that p2 is transiently required for proper assembly, but needs to be removed from the C terminus of CA to weaken CA-CA interactions and allow the rearrangement of the virion core shell during virus maturation.
Figures

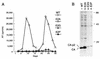

References
-
- Accola, M. A., and H. G. Göttlinger. Unpublished observation.
-
- Blaber M, Zhang X-J, Matthews B W. Structural basis of amino acid α helix propensity. Science. 1993;260:1637–1640. - PubMed
-
- Bryson J W, Betz S F, Lu H S, Suich D J, Zhou H X, O’Neil K T, DeGrado W F. Protein design: a hierarchic approach. Science. 1995;270:935–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

