Signal peptidase cleavage at the flavivirus C-prM junction: dependence on the viral NS2B-3 protease for efficient processing requires determinants in C, the signal peptide, and prM
- PMID: 9499070
- PMCID: PMC109509
- DOI: 10.1128/JVI.72.3.2141-2149.1998
Signal peptidase cleavage at the flavivirus C-prM junction: dependence on the viral NS2B-3 protease for efficient processing requires determinants in C, the signal peptide, and prM
Abstract
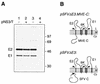
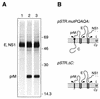
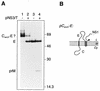
Signal peptidase cleavage at the C-prM junction in the flavivirus structural polyprotein is inefficient in the absence of the cytoplasmic viral protease, which catalyzes cleavage at the COOH terminus of the C protein. The signal peptidase cleavage occurs efficiently in circumstances where the C protein is deleted or if the viral protease complex is present. In this study, we used cDNA of Murray Valley encephalitis virus (MVE) to examine features of the structural polyprotein which allow this regulation of a luminal cleavage by a cytoplasmic protease. We found that the inefficiency of signal peptidase cleavage in the absence of the viral protease is not attributable solely to features of the C protein. Inhibition of cleavage still occurred when charged residues in C were mutated to uncharged residues or when an unrelated protein sequence (that of ubiquitin) was substituted for C. Also, fusion of the C protein did not inhibit processing of an alternative adjacent signal sequence. The cleavage region of the flavivirus prM translocation signal is unusually hydrophobic, and we established that altering this characteristic by making three point mutations near the signal peptidase cleavage site in MVE prM dramatically increased the extent of cleavage without requiring removal of the C protein. In addition, we demonstrated that luminal sequences downstream from the signal peptidase cleavage site contributed to the inefficiency of cleavage.
Figures





Similar articles
-
Formation of the flavivirus envelope: role of the viral NS2B-NS3 protease.J Virol. 1995 Apr;69(4):1995-2003. doi: 10.1128/JVI.69.4.1995-2003.1995. J Virol. 1995. PMID: 7884844 Free PMC article.
-
NS2B/3 proteolysis at the C-prM junction of the tick-borne encephalitis virus polyprotein is highly membrane dependent.Virus Res. 2012 Sep;168(1-2):48-55. doi: 10.1016/j.virusres.2012.06.012. Epub 2012 Jun 19. Virus Res. 2012. PMID: 22727684 Free PMC article.
-
Mutagenesis of the signal sequence of yellow fever virus prM protein: enhancement of signalase cleavage In vitro is lethal for virus production.J Virol. 2000 Jan;74(1):24-32. doi: 10.1128/jvi.74.1.24-32.2000. J Virol. 2000. PMID: 10590087 Free PMC article.
-
Delayed by Design: Role of Suboptimal Signal Peptidase Processing of Viral Structural Protein Precursors in Flaviviridae Virus Assembly.Viruses. 2020 Sep 26;12(10):1090. doi: 10.3390/v12101090. Viruses. 2020. PMID: 32993149 Free PMC article. Review.
-
Post-translational regulation and modifications of flavivirus structural proteins.J Gen Virol. 2015 Jul;96(Pt 7):1551-69. doi: 10.1099/vir.0.000097. Epub 2015 Feb 23. J Gen Virol. 2015. PMID: 25711963 Review.
Cited by
-
The West Nile virus capsid protein blocks apoptosis through a phosphatidylinositol 3-kinase-dependent mechanism.J Virol. 2013 Jan;87(2):872-81. doi: 10.1128/JVI.02030-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115297 Free PMC article.
-
Stable high-producer cell clone expressing virus-like particles of the Japanese encephalitis virus e protein for a second-generation subunit vaccine.J Virol. 2003 Aug;77(16):8745-55. doi: 10.1128/jvi.77.16.8745-8755.2003. J Virol. 2003. PMID: 12885894 Free PMC article.
-
Role of Capsid Anchor in the Morphogenesis of Zika Virus.J Virol. 2018 Oct 29;92(22):e01174-18. doi: 10.1128/JVI.01174-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158295 Free PMC article.
-
Nuclear localization of Japanese encephalitis virus core protein enhances viral replication.J Virol. 2005 Mar;79(6):3448-58. doi: 10.1128/JVI.79.6.3448-3458.2005. J Virol. 2005. PMID: 15731239 Free PMC article.
-
Construction and mutagenesis of an artificial bicistronic tick-borne encephalitis virus genome reveals an essential function of the second transmembrane region of protein e in flavivirus assembly.J Virol. 2006 Dec;80(24):12197-208. doi: 10.1128/JVI.01540-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035331 Free PMC article.
References
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
-
- Baker R T, Smith S A, Marano R, McKee J, Board P G. Protein expression using cotranslational fusion and cleavage of ubiquitin. J Biol Chem. 1994;269:25381–25386. - PubMed
-
- Brodsky J L. Post-translational protein translocation: not all hsc70s are created equal. Trends Biochem Sci. 1996;21:122–126. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

