Molecular characterization of hemagglutination domains on the fibers of subgenus D adenoviruses
- PMID: 9499089
- PMCID: PMC109528
- DOI: 10.1128/JVI.72.3.2297-2304.1998
Molecular characterization of hemagglutination domains on the fibers of subgenus D adenoviruses
Abstract
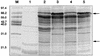
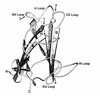
The adenovirus fiber mediates the agglutination of erythrocytes. Based on differential hemagglutinating properties, subgenus D adenoviruses can be subdivided into clusters DI, DII, and DIII. While subgenus DI adenoviruses agglutinate rat and human erythrocytes, DII adenoviruses simply agglutinate rat erythrocytes and DIII adenoviruses display no or only weak rat erythrocyte agglutination. Amino acid sequence comparisons revealed distinct domains on the fiber knob which could be involved in hemagglutination. In order to localize and characterize the domains responsible for the interaction with rat and human erythrocytes, potential hemagglutination domains of the adenovirus type 9 (Ad9) (subgenus DI) fiber knob were introduced into Ad17 (subgenus DII) and Ad28 (subgenus DIII) fiber knobs by primer-directed mutagenesis. Furthermore, rat erythrocyte hemagglutination domains were also introduced into the Ad3 (subgenus B) fiber knob, which only agglutinated monkey erythrocytes. Altogether, 27 chimeric and mutated fiber proteins were expressed in Escherichia coli and subsequently tested for hemagglutination activity. The hemagglutination tests revealed that at least two domains can mediate the agglutination of rat erythrocytes. While one domain is located on the GH loop, the other domain extends from the C beta strand to the CD loop. The domain on the GH loop was partially conserved in all adenoviruses showing an incomplete hemagglutination pattern with rat erythrocytes. The domains involved in the agglutination of human erythrocytes are located on the CD and HI loops of the subgenus DI fiber knob.
Figures



Similar articles
-
Molecular characterization of the type-specific gamma-determinant located on the adenovirus fiber.J Virol. 1997 Sep;71(9):6576-81. doi: 10.1128/JVI.71.9.6576-6581.1997. J Virol. 1997. PMID: 9261379 Free PMC article.
-
Recombinant fibre proteins of human adenoviruses Ad9, Ad15 and Ad19: localization of the haemagglutination properties and the type-specific determinant.Res Virol. 1997 Jan-Feb;148(1):5-10. doi: 10.1016/s0923-2516(97)81905-x. Res Virol. 1997. PMID: 9017826
-
Adenovirus serotype 30 fiber does not mediate transduction via the coxsackie-adenovirus receptor.J Virol. 2002 Jan;76(2):656-61. doi: 10.1128/jvi.76.2.656-661.2002. J Virol. 2002. PMID: 11752156 Free PMC article.
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
-
Adenovirus interaction with its cellular receptor CAR.Curr Top Microbiol Immunol. 2003;272:331-64. doi: 10.1007/978-3-662-05597-7_11. Curr Top Microbiol Immunol. 2003. PMID: 12747555 Review.
Cited by
-
Adenovirus type 37 uses sialic acid as a cellular receptor.J Virol. 2000 Jan;74(1):42-8. J Virol. 2000. PMID: 10590089 Free PMC article.
-
Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector.J Virol. 2000 Mar;74(6):2567-83. doi: 10.1128/jvi.74.6.2567-2583.2000. J Virol. 2000. PMID: 10684271 Free PMC article.
-
Adenovirus type 11 uses CD46 as a cellular receptor.J Virol. 2003 Sep;77(17):9183-91. doi: 10.1128/jvi.77.17.9183-9191.2003. J Virol. 2003. PMID: 12915534 Free PMC article.
-
The Arg279Gln [corrected] substitution in the adenovirus type 11p (Ad11p) fiber knob abolishes EDTA-resistant binding to A549 and CHO-CD46 cells, converting the phenotype to that of Ad7p.J Virol. 2006 Feb;80(4):1897-905. doi: 10.1128/JVI.80.4.1897-1905.2006. J Virol. 2006. PMID: 16439545 Free PMC article.
-
Dependence of adenovirus infectivity on length of the fiber shaft domain.J Virol. 2000 Nov;74(22):10274-86. doi: 10.1128/jvi.74.22.10274-10286.2000. J Virol. 2000. PMID: 11044071 Free PMC article.
References
-
- Arnberg N, Mei Y-F, Wadell G. Fiber genes of adenoviruses with tropism for the eye and the genital tract. Virology. 1997;227:239–244. - PubMed
-
- Bauer H, Wigand R. Eigenschaften der Adenovirus-Hämagglutinine. Z Hyg Infektionskr. 1963;149:96. - PubMed
-
- Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Chroboczek J, Ruigrok R W H, Cusack S. Adenovirus fiber. In: Doerfler W, Boehm P, editors. The molecular repertoire of adenoviruses. I. Berlin, Germany: Springer-Verlag; 1995. pp. 163–200.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

