Characterization of an autonomous subgenomic pestivirus RNA replicon
- PMID: 9499097
- PMCID: PMC109536
- DOI: 10.1128/JVI.72.3.2364-2372.1998
Characterization of an autonomous subgenomic pestivirus RNA replicon
Abstract
As an initial approach to define the requirements for the replication of bovine viral diarrhea virus (BVDV), a member of the Flaviviridae family with a positive-strand RNA genome, full-length genomic and subgenomic RNAs were originated by in vitro transcription of diverse BVDV cDNA constructs and transfected into eucaryotic host cells. RNA replication was measured either directly by an RNase protection method or by monitoring the synthesis of viral protein. When full-length BVDV cRNA was initially applied, the synthesis of negative-strand RNA intermediates as well as progeny positive-strand RNA was detected posttransfection in the cytoplasm of the host cells. Compared to the negative-strand RNA intermediate, an excess of positive-strand RNA was synthesized. Surprisingly, a subgenomic RNA molecule, DI9c, corresponding to a previously characterized defective interfering particle, was found to support both steps of RNA replication in the absence of a helper virus as well, thus functioning as an autonomous replicon. DI9c comprises the 5' and 3' untranslated regions of the BVDV genome and the coding regions of the autoprotease Npro and the nonstructural proteins NS3, NS4A, NS4B, NS5A, and NS5B. Most interestingly, the NS2 polypeptide was thus determined to be nonessential for RNA replication. As expected, deletion of the genomic 3' end as well as abolition of the catalytic function of the virus-encoded serine protease resulted in DI9c molecules that were unable to replicate. Deletion of the entire Npro gene also destroyed the ability of DI9c molecules to replicate. On the other hand, DI9c derivatives in which the 5' third of the Npro gene was fused to a ubiquitin gene, allowing the proteolytic release of NS3 in trans, turned out to be replication competent. These results suggest that the RNA sequence located at the 5' end of the open reading frame exerts an essential role during BVDV replication. Replication of DI9c and DI9c derivatives was found not to be limited to host cells of bovine origin, indicating that cellular factors functioning as potential parts of the viral replication machinery are well conserved between different mammalian cells. Our data provide an important step toward the ready identification and characterization of viral factors and genomic elements involved in the life cycle of pestiviruses. The implications for other Flaviviridae and, in particular, the BVDV-related human hepatitis C virus are discussed.
Figures


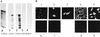

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

