African swine fever virus is wrapped by the endoplasmic reticulum
- PMID: 9499098
- PMCID: PMC109537
- DOI: 10.1128/JVI.72.3.2373-2387.1998
African swine fever virus is wrapped by the endoplasmic reticulum
Abstract
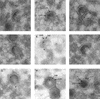
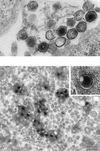
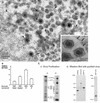
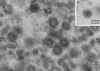
African swine fever (ASF) virus is a large DNA virus that shares the striking icosahedral symmetry of iridoviruses and the genomic organization of poxviruses. Both groups of viruses have a complex envelope structure. In this study, the mechanism of formation of the inner envelope of ASF virus was investigated. Examination of thin cryosections by electron microscopy showed two internal membranes in mature intracellular virions and all structural intermediates. These membranes were in continuity with intracellular membrane compartments, suggesting that the virus gained two membranes from intracellular membrane cisternae. Immunogold electron microscopy showed the viral structural protein p17 and resident membrane proteins of the endoplasmic reticulum (ER) within virus assembly sites, virus assembly intermediates, and mature virions. Resident ER proteins were also detected by Western blotting of isolated virions. The data suggested the ASF virus was wrapped by the ER. Analysis of the published sequence of ASF virus (R. J. Yanez et al., Virology 208:249-278, 1995) revealed a reading frame, XP124L, that encoded a protein predicted to translocate into the lumen of the ER. Pulse-chase immunoprecipitation and glycosylation analysis of pXP124L, the product of the XP124L gene, showed that pXP124L was retained in the ER lumen after synthesis. When analyzed by immunogold electron microscopy, pXP124L localized to virus assembly intermediates and fully assembled virions. Western blot analysis detected pXP124L in virions isolated from Percoll gradients. The packaging of pXP124L from the lumen of the ER into the virion is consistent with ASF virus being wrapped by ER cisternae: a mechanism which explains the presence of two membranes in the viral envelope.
Figures

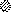 ,
membrane glycoproteins). Interactions between viral proteins lead to
membrane curvature, and the virion gains a single membrane by budding
into the lumen of the membrane compartment. When the virion is released
from the cell, oligosaccharides (
,
membrane glycoproteins). Interactions between viral proteins lead to
membrane curvature, and the virion gains a single membrane by budding
into the lumen of the membrane compartment. When the virion is released
from the cell, oligosaccharides (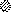 ) are exposed on the surface of the
virus, and the cytoplasmic tail of the membrane glycoprotein is buried
within the virion. (b) Wrapping. Viral nucleoprotein complexes bind to
the cytoplasmic domains of virally encoded integral membrane proteins.
The nucleoprotein complex is then wrapped by the membrane cisternae,
and the virus gains two membranes. The particle remains in the cytosol.
When the virion is released from the cell by cell lysis,
oligosaccharides (
) are exposed on the surface of the
virus, and the cytoplasmic tail of the membrane glycoprotein is buried
within the virion. (b) Wrapping. Viral nucleoprotein complexes bind to
the cytoplasmic domains of virally encoded integral membrane proteins.
The nucleoprotein complex is then wrapped by the membrane cisternae,
and the virus gains two membranes. The particle remains in the cytosol.
When the virion is released from the cell by cell lysis,
oligosaccharides (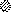 ) are buried within the two membranes of the
virion while the cytoplasmic tail of the membrane glycoprotein is
exposed on the surface of the virus.
) are buried within the two membranes of the
virion while the cytoplasmic tail of the membrane glycoprotein is
exposed on the surface of the virus.



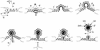
References
-
- Alves De Matos A P, Marcal M R, Moura Nunes F J, Castro Portugal F L, Vigario J D. Ultrastructural and cytochemical aspects of the maturation of the ASFV particle. Rep Trab Inst Nac Vet. 1980;12:79–84.
-
- Arzuza O, Urzainqui A, Diaz-Ruiz J R, Tabares E. Morphogenesis of African swine fever virus in monkey kidney cells after reversible inhibition of replication by cycloheximide. Arch Virol. 1992;124:343–354. - PubMed
-
- Black D N, Brown F. Purification and physicochemical characteristics of ASFV. J Gen Virol. 1976;32:509–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

