The extracellular domain of vaccinia virus protein B5R affects plaque phenotype, extracellular enveloped virus release, and intracellular actin tail formation
- PMID: 9499104
- PMCID: PMC109543
- DOI: 10.1128/JVI.72.3.2429-2438.1998
The extracellular domain of vaccinia virus protein B5R affects plaque phenotype, extracellular enveloped virus release, and intracellular actin tail formation
Abstract
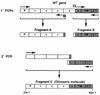
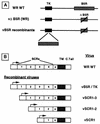
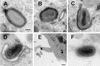
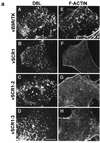
Vaccinia virus produces two morphologically distinct forms of infectious virus, termed intracellular mature virus (IMV) and extracellular enveloped virus (EEV). EEV is important for virus dissemination within a host and has different surface proteins which bind to cell receptors different from those used by IMV. Six genes are known to encode EEV-specific proteins. One of these, B5R, encodes a 42-kDa glycoprotein with amino acid similarity to members of the complement control protein superfamily and contains four copies of a 50- to 70-amino-acid repeat called the short consensus repeat (SCR). Deletion of B5R causes a small-plaque phenotype, a 10-fold reduction in EEV formation, and virus attenuation in vivo. In this study, we inserted mutated versions of the B5R gene lacking different combinations of the SCRs into a virus deletion mutant lacking the B5R gene. The resultant viruses each formed small plaques only slightly larger than those of the deletion mutant; however, the virus containing only SCR 1 formed plaques slightly larger than those of viruses with SCRs 1 and 2 or SCRs 1, 2, and 3. All of these viruses produced approximately 50-fold more infectious EEV than wild-type virus and formed comet-shaped plaques under liquid overlay. Despite producing more EEV, the mutant viruses were unable to induce the polymerization of actin on intracellular virus particles. The implications of these results for our understanding of EEV formation, release, and infectivity are discussed.
Figures



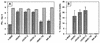
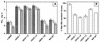


Similar articles
-
Functional analysis of vaccinia virus B5R protein: essential role in virus envelopment is independent of a large portion of the extracellular domain.J Virol. 1998 Jan;72(1):294-302. doi: 10.1128/JVI.72.1.294-302.1998. J Virol. 1998. PMID: 9420227 Free PMC article.
-
Replacing the SCR domains of vaccinia virus protein B5R with EGFP causes a reduction in plaque size and actin tail formation but enveloped virions are still transported to the cell surface.J Gen Virol. 2002 Feb;83(Pt 2):323-332. doi: 10.1099/0022-1317-83-2-323. J Gen Virol. 2002. PMID: 11807225
-
A mutational analysis of the vaccinia virus B5R protein.J Gen Virol. 2001 May;82(Pt 5):1199-1213. doi: 10.1099/0022-1317-82-5-1199. J Gen Virol. 2001. PMID: 11297695
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
Cited by
-
Murine gammaherpesvirus 68 encodes a functional regulator of complement activation.J Virol. 1999 Sep;73(9):7658-70. doi: 10.1128/JVI.73.9.7658-7670.1999. J Virol. 1999. PMID: 10438856 Free PMC article.
-
Suppression of NYVAC Infection in HeLa Cells Requires RNase L but Is Independent of Protein Kinase R Activity.J Virol. 2015 Dec 9;90(4):2135-41. doi: 10.1128/JVI.02576-15. Print 2016 Feb 15. J Virol. 2015. PMID: 26656695 Free PMC article.
-
A systemically deliverable Vaccinia virus with increased capacity for intertumoral and intratumoral spread effectively treats pancreatic cancer.J Immunother Cancer. 2021 Jan;9(1):e001624. doi: 10.1136/jitc-2020-001624. J Immunother Cancer. 2021. PMID: 33500259 Free PMC article.
-
Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R.J Virol. 2005 May;79(10):6260-71. doi: 10.1128/JVI.79.10.6260-6271.2005. J Virol. 2005. PMID: 15858010 Free PMC article.
-
Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera.J Virol. 2001 May;75(10):4802-13. doi: 10.1128/JVI.75.10.4802-4813.2001. J Virol. 2001. PMID: 11312352 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

