The M2 ectodomain is important for its incorporation into influenza A virions
- PMID: 9499106
- PMCID: PMC109545
- DOI: 10.1128/JVI.72.3.2449-2455.1998
The M2 ectodomain is important for its incorporation into influenza A virions
Abstract
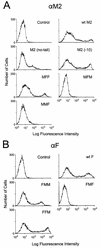
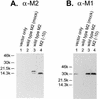
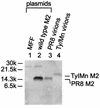
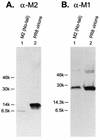
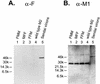
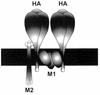
M2 is an integral protein of influenza A virus that functions as an ion channel. The ratio of M2 to HA in influenza A virions differs from that found on the cell surface, suggesting selective incorporation of M2 and HA into influenza virions. To examine the sequences that are important for M2 incorporation into virions, we used an incorporation assay that involves expressing M2 from a plasmid, transfecting the plasmid into recipient cells, and then infecting those cells with influenza virus. To test the importance of the different regions of the protein (extracellular, transmembrane, and cytoplasmic) in determining M2 incorporation, we created chimeric mutants of M2 and Sendai virus F proteins, exchanging corresponding extracellular, transmembrane, and cytoplasmic domains. Of the six possible chimeric mutants, only three were expressed on the cell surface. Of these three chimeric proteins, only one mutant (with the extracellular domain from M2 and the rest from F) was incorporated into influenza virions. These results suggest that the extracellular domain of M2 is important for its incorporation into virions.
Figures

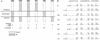

References
-
- Arcus Y M, Knight C A. Properties of host components of PR8 influenza virus. Intervirology. 1981;15:171–176. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1995.
-
- Blumberg B M, Giorgi C, Rose K, Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985;66:317–331. - PubMed
-
- Bousse T, Takimoto T, Gorman W L, Takahashi T, Portner A. Regions on the hemagglutinin-neuraminidase proteins of human parainfluenza virus type-1 and Sendai virus important for membrane fusion. Virology. 1994;204:506–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

