The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments
- PMID: 9499108
- PMCID: PMC109547
- DOI: 10.1128/JVI.72.3.2463-2473.1998
The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments
Abstract
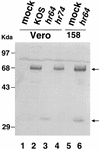
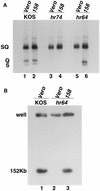
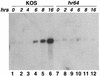
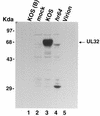
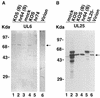
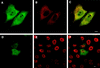
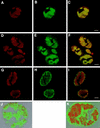
Six genes, including UL32, have been implicated in the cleavage and packaging of herpesvirus DNA into preassembled capsids. We have isolated a UL32 insertion mutant which is capable of near-wild-type levels of viral DNA synthesis; however, the mutant virus is unable to cleave and package viral DNA, consistent with the phenotype of a previously isolated temperature-sensitive herpes simplex virus type 1 mutant, tsN20 (P. A. Schaffer, G. M. Aron, N. Biswal, and M. Benyesh-Melnick, Virology 52:57-71, 1973). A polyclonal antibody which recognizes UL32 was previously used by Chang et al. (Y. E. Chang, A. P. Poon, and B. Roizman, J. Virol. 70:3938-3946, 1996) to demonstrate that UL32 accumulates predominantly in the cytoplasm of infected cells. In this report, a functional epitope-tagged version of UL32 showed that while UL32 is predominantly cytoplasmic, some nuclear staining which colocalizes with the major DNA binding protein (ICP8, UL29) in replication compartments can be detected. We have also used a monoclonal antibody (5C) specific for the hexon form of major capsid protein VP5 to study the distribution of capsids during infection. In cells infected with wild-type KOS (6 and 8 h postinfection), 5C staining patterns indicate that capsids are present in nuclei within replication compartments. These results suggest that cleavage and packaging occur in replication compartments at least at 6 and 8 h postinfection. Cells infected with the UL32 mutant exhibit a hexon staining pattern which is more diffusely distributed throughout the nucleus and which is not restricted to replication compartments. We propose that UL32 may play a role in "bringing" preassembled capsids to the sites of DNA packaging and that the failure to localize to replication compartments may explain the cleavage/packaging defect exhibited by this mutant. These results suggest that the UL32 protein is required at a step distinct from those at which other cleavage and packaging proteins are required and may be involved in the correct localization of capsids within infected cells.
Figures

Similar articles
-
The herpes simplex virus 1 UL 17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged.Virology. 1998 Dec 5;252(1):115-25. doi: 10.1006/viro.1998.9439. Virology. 1998. PMID: 9875322
-
The putative herpes simplex virus 1 chaperone protein UL32 modulates disulfide bond formation during infection.J Virol. 2015 Jan;89(1):443-53. doi: 10.1128/JVI.01913-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320327 Free PMC article.
-
Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging.J Virol. 1998 Sep;72(9):7428-39. doi: 10.1128/JVI.72.9.7428-7439.1998. J Virol. 1998. PMID: 9696839 Free PMC article.
-
Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins.J Virol. 1997 Apr;71(4):2656-65. doi: 10.1128/JVI.71.4.2656-2665.1997. J Virol. 1997. PMID: 9060618 Free PMC article.
-
Procapsid assembly, maturation, nuclear exit: dynamic steps in the production of infectious herpesvirions.Adv Exp Med Biol. 2012;726:423-39. doi: 10.1007/978-1-4614-0980-9_19. Adv Exp Med Biol. 2012. PMID: 22297525 Free PMC article. Review.
Cited by
-
Proteolytic cleavage of the amino terminus of the U(L)15 gene product of herpes simplex virus type 1 is coupled with maturation of viral DNA into unit-length genomes.J Virol. 1999 Oct;73(10):8338-48. doi: 10.1128/JVI.73.10.8338-8348.1999. J Virol. 1999. PMID: 10482584 Free PMC article.
-
Packaging of genomic and amplicon DNA by the herpes simplex virus type 1 UL25-null mutant KUL25NS.J Virol. 2001 Nov;75(22):10755-65. doi: 10.1128/JVI.75.22.10755-10765.2001. J Virol. 2001. PMID: 11602717 Free PMC article.
-
DNA cleavage and packaging proteins encoded by genes U(L)28, U(L)15, and U(L)33 of herpes simplex virus type 1 form a complex in infected cells.J Virol. 2002 May;76(10):4785-91. doi: 10.1128/jvi.76.10.4785-4791.2002. J Virol. 2002. PMID: 11967295 Free PMC article.
-
Quantification of the DNA cleavage and packaging proteins U(L)15 and U(L)28 in A and B capsids of herpes simplex virus type 1.J Virol. 2004 Feb;78(3):1367-74. doi: 10.1128/jvi.78.3.1367-1374.2004. J Virol. 2004. PMID: 14722291 Free PMC article.
-
Analysis of HCF, the cellular cofactor of VP16, in herpes simplex virus-infected cells.J Virol. 2000 Jan;74(1):99-109. doi: 10.1128/jvi.74.1.99-109.2000. J Virol. 2000. PMID: 10590096 Free PMC article.
References
-
- Addison C, Rixon F J, Palfreyman J W, O’Hara M, Preston V G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984;138:246–259. - PubMed
-
- Addison C, Rixon F J, Preston V G. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J Gen Virol. 1990;71:2377–2384. - PubMed
-
- Ali M A, Forghani B, Cantin E M. Characterization of an essential HSV-1 protein encoded by the UL25 gene reported to be involved in virus penetration and capsid assembly. Virology. 1996;216:278–283. - PubMed
-
- Al-Kobaisi M F, Rixon F J, McDougall I, Preston V G. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991;180:380–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

