Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes
- PMID: 9500790
- PMCID: PMC2212187
- DOI: 10.1084/jem.187.6.875
Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes
Abstract
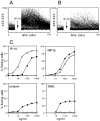
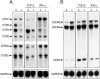
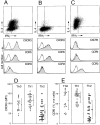
Chemokines and their receptors are important elements for the selective attraction of various subsets of leukocytes. To better understand the selective migration of functional subsets of T cells, chemokine receptor expression was analyzed using monoclonal antibodies, RNase protection assays, and the response to distinct chemokines. Naive T cells expressed only CXC chemokine receptor (CXCR)4, whereas the majority of memory/activated T cells expressed CXCR3, and a small proportion expressed CC chemokine receptor (CCR)3 and CCR5. When polarized T cell lines were analyzed, CXCR3 was found to be expressed at high levels on T helper cell (Th)0s and Th1s and at low levels on Th2s. In contrast, CCR3 and CCR4 were found on Th2s. This was confirmed by functional responses: only Th2s responded with an increase in [Ca2+]i to the CCR3 and CCR4 agonists eotaxin and thymus and activation regulated chemokine (TARC), whereas only Th0s and Th1s responded to low concentrations of the CXCR3 agonists IFN-gamma-inducible protein 10 (IP-10) and monokine induced by IFN-gamma (Mig). Although CCR5 was expressed on both Th1 and Th2 lines, it was absent in several Th2 clones and its expression was markedly influenced by interleukin 2. Chemokine receptor expression and association with Th1 and Th2 phenotypes was affected by other cytokines present during polarization. Transforming growth factor beta inhibited CCR3, but enhanced CCR4 and CCR7 expression, whereas interferon alpha inhibited CCR3 but upregulated CXCR3 and CCR1. These results demonstrate that chemokine receptors are markers of naive and polarized T cell subsets and suggest that flexible programs of chemokine receptor gene expression may control tissue-specific migration of effector T cells.
Figures




References
-
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
-
- Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. - PubMed
-
- Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–427. - PubMed
-
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

