Specific peptide-activated proteolytic cleavage of Escherichia coli elongation factor Tu
- PMID: 9501186
- PMCID: PMC19665
- DOI: 10.1073/pnas.95.6.2891
Specific peptide-activated proteolytic cleavage of Escherichia coli elongation factor Tu
Abstract
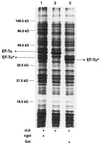
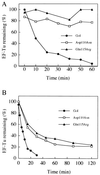
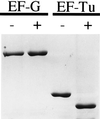
Phage exclusion is a form of programmed cell death in prokaryotes in which death is triggered by infection with phage, a seemingly altruistic response that limits multiplication of the phage and its spread through the population. One of the best-characterized examples of phage exclusion is the exclusion of T-even phages such as T4 by the e14-encoded Lit protein in many Escherichia coli K-12 strains. In this exclusion system, transcription and translation of a short region of the major head coat protein gene late in phage infection activates proteolysis of translation elongation factor Tu (EF-Tu), blocking translation and multiplication of the phage. The cleavage occurs between Gly-59 and Ile-60 in the nucleotide-binding domain. In the present work, we show that a 29-residue synthetic peptide spanning the activating region of the major head coat protein can activate the cleavage of GDP-bound EF-Tu in a purified system containing only purified EF-Tu and purified Lit protein. Lit behaves as a bona fide enzyme in this system, cleaving EF-Tu to completion when present at substoichiometric amounts. Two mutant peptides with amino acid changes that reduce the activation of cleavage of EF-Tu in vivo were also greatly reduced in their ability to activate EF-Tu cleavage in vitro but were still able to activate cleavage at a high concentration. Elongation factor G, which has the same sequence at the cleavage site and a nucleotide-binding domain similar to EF-Tu, was not cleaved by this system, and neither was heat-inactivated EF-Tu, suggesting that the structural context of the cleavage site may be important for specificity. This system apparently represents an activation mechanism for proteolysis that targets one of nature's most evolutionarily conserved proteins for site-specific cleavage.
Figures

Comment in
-
The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death.Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):2727-30. doi: 10.1073/pnas.95.6.2727. Proc Natl Acad Sci U S A. 1998. PMID: 9501156 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

