Transport of the new chemotherapeutic agent beta-D-glucosylisophosphoramide mustard (D-19575) into tumor cells is mediated by the Na+-D-glucose cotransporter SAAT1
- PMID: 9501190
- PMCID: PMC19669
- DOI: 10.1073/pnas.95.6.2914
Transport of the new chemotherapeutic agent beta-D-glucosylisophosphoramide mustard (D-19575) into tumor cells is mediated by the Na+-D-glucose cotransporter SAAT1
Abstract
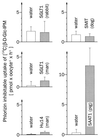
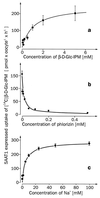
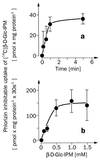
For beta-D-glucosylisophosphoramide mustard (beta-D-Glc-IPM), a new alkylating drug in which isophosphoramide mustard is stabilized, a higher selectivity and lower myelotoxicity was observed than for the currently used cytostatic ifosfamide. Because beta-D-Glc-IPM is hydrophilic and does not diffuse passively through the lipid bilayer, we investigated whether a transporter may be involved in the cellular uptake. A variety of cloned Na+-sugar cotransporters were expressed in Xenopus oocytes, and uptake measurements were performed. By tracer uptake and electrical measurements it was found that beta-D-Glc-IPM was transported by the low-affinity Na+-D-glucose cotransporter SAAT1, which had been cloned from pig and is also expressed in humans. At membrane potentials between -50 and -150 mV, a 10-fold higher substrate affinity (Km approximately 0.25 mM) and a 10-fold lower Vmax value were estimated for beta-D-Glc-IPM transport than for the transport of D-glucose or methyl-alpha-D-glucopyranoside (AMG). Transport of beta-D-Glc-IPM and glucose by SAAT1 is apparently performed by the same mechanism because similar sodium dependence, dependence on membrane potential, electrogenicity, and phlorizin inhibition were determined for beta-D-Glc-IPM, D-glucose, and AMG. Transcription of human SAAT1 was demonstrated in various human carcinomas and tumor cell lines. In one of these, the human carcinoma cell line T84, phlorizin inhibitable uptake of beta-D-Glc-IPM was demonstrated with substrate saturation and an apparent Km of 0.4 mM. The data suggest that the Na+-D-glucose cotransporter SAAT1 transports beta-D-Glc-IPM into human tumor cells and may accumulate the drug in the cells. They provide an example for drug targeting by employing a plasma membrane transporter.
Figures




References
-
- Schwartsmann G, Workman P. Eur J Cancer. 1993;29A:3–14. - PubMed
-
- Tietze, L. F., Fischer, R., Beller, M. & Seele, R. (1990) Liebigs Ann. Chem. 151–157.
-
- Schwabe K, Graffi A, Redslob J, Hülsmann W, Butschak G. Pharmazie. 1976;31:438–441. - PubMed
-
- Pohl J, Bertram B, Hilgard P, Nowrousian M R, Stüben J, Wießler M. Cancer Chemother Pharmacol. 1995;35:364–370. - PubMed
-
- Hediger M A, Rhoads D B. Physiol Rev. 1994;74:993–1026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

