Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel
- PMID: 9501192
- PMCID: PMC19671
- DOI: 10.1073/pnas.95.6.2926
Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel
Abstract
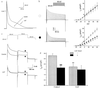
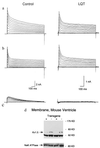
Voltage-gated potassium channels control cardiac repolarization, and mutations of K+ channel genes recently have been shown to cause arrhythmias and sudden death in families with the congenital long QT syndrome. The precise mechanism by which the mutations lead to QT prolongation and arrhythmias is uncertain, however. We have shown previously that an N-terminal fragment including the first transmembrane segment of the rat delayed rectifier K+ channel Kv1.1 (Kv1.1N206Tag) coassembles with other K+ channels of the Kv1 subfamily in vitro, inhibits the currents encoded by Kv1.5 in a dominant-negative manner when coexpressed in Xenopus oocytes, and traps Kv1.5 polypeptide in the endoplasmic reticulum of GH3 cells. Here we report that transgenic mice overexpressing Kv1.1N206Tag in the heart have a prolonged QT interval and ventricular tachycardia. Cardiac myocytes from these mice have action potential prolongation caused by a significant reduction in the density of a rapidly activating, slowly inactivating, 4-aminopyridine sensitive outward K+ current. These changes correlate with a marked decrease in the level of Kv1.5 polypeptide. Thus, overexpression of a truncated K+ channel in the heart alters native K+ channel expression and has profound effects on cardiac excitability.
Figures


References
-
- Curran M E, Splawski I, Timothy K W, Vincent G M, Green E D, Keating M T. Cell. 1995;80:795–803. - PubMed
-
- Wang Q, Curran M E, Splawski I, Burn T C, Millholland J M, Van Raay T J, Shen J, Timothy K W, Vincent G M, de Jager T, et al. Nat Genet. 1996;12:17–23. - PubMed
-
- Sanguinetti M C, Jiang C, Curran M E, Keating M T. Cell. 1995;81:299–307. - PubMed
-
- Trudeau M C, Waronhe J M, Ganetsky B, Robertson G A. Science. 1995;269:92–95. - PubMed
-
- Sanguinetti M C, Curran M E, Zou A, Shen J, Spector P S, Atkinson D L, Keating M T. Nature (London) 1996;384:80–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

