A large and distinct rotation of the myosin light chain domain occurs upon muscle contraction
- PMID: 9501195
- PMCID: PMC19674
- DOI: 10.1073/pnas.95.6.2944
A large and distinct rotation of the myosin light chain domain occurs upon muscle contraction
Abstract
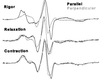
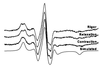
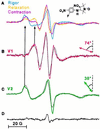
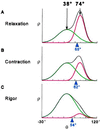
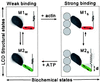
For more than 30 years, the fundamental goal in molecular motility has been to resolve force-generating motor protein structural changes. Although low-resolution structural studies have provided evidence for force-generating myosin rotations upon muscle activation, these studies did not resolve structural states of myosin in contracting muscle. Using electron paramagnetic resonance, we observed two distinct orientations of a spin label attached specifically to a single site on the light chain domain of myosin in relaxed scallop muscle fibers. The two probe orientations, separated by a 36 degrees +/- 5 degrees axial rotation, did not change upon muscle activation, but the distribution between them changed substantially, indicating that a fraction (17% +/- 2%) of myosin heads undergoes a large (at least 30 degrees) axial rotation of the myosin light chain domain upon force generation and muscle contraction. The resulting model helps explain why this observation has remained so elusive and provides insight into the mechanisms by which motor protein structural transitions drive molecular motility.
Figures
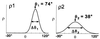
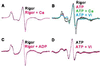

Comment in
-
New angle on myosin.Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):2720-2. doi: 10.1073/pnas.95.6.2720. Proc Natl Acad Sci U S A. 1998. PMID: 9501153 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

