HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte
- PMID: 9501201
- PMCID: PMC19680
- DOI: 10.1073/pnas.95.6.2979
HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte
Abstract
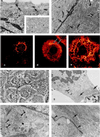
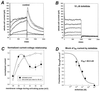
We have derived a cardiac muscle cell line, designated HL-1, from the AT-1 mouse atrial cardiomyocyte tumor lineage. HL-1 cells can be serially passaged, yet they maintain the ability to contract and retain differentiated cardiac morphological, biochemical, and electrophysiological properties. Ultrastructural characteristics typical of embryonic atrial cardiac muscle cells were found consistently in the cultured HL-1 cells. Reverse transcriptase-PCR-based analyses confirmed a pattern of gene expression similar to that of adult atrial myocytes, including expression of alpha-cardiac myosin heavy chain, alpha-cardiac actin, and connexin43. They also express the gene for atrial natriuretic factor. Immunohistochemical staining of the HL-1 cells indicated that the distribution of the cardiac-specific markers desmin, sarcomeric myosin, and atrial natriuretic factor was similar to that of cultured atrial cardiomyocytes. A delayed rectifier potassium current (IKr) was the most prominent outward current in HL-1 cells. The activating currents displayed inward rectification and deactivating current tails were voltage-dependent, saturated at >>+20 mV, and were highly sensitive to dofetilide (IC50 of 46.9 nM). Specific binding of [3H]dofetilide was saturable and fit a one-site binding isotherm with a Kd of 140 +/- 60 nM and a Bmax of 118 fmol per 10(5) cells. HL-1 cells represent a cardiac myocyte cell line that can be repeatedly passaged and yet maintain a cardiac-specific phenotype.
Figures

References
-
- Field L J. Science. 1988;239:1029–1033. - PubMed
-
- Steinhelper M E, Lanson N A, Jr, Dresdner K P, Delcarpio J B, Wit A L, Claycomb W C, Field L J. Am J Physiol. 1990;259:H1826–H1834. - PubMed
-
- Delcarpio J B, Lanson N A, Jr, Field L J, Claycomb W C. Circ Res. 1991;69:1591–1600. - PubMed
-
- Lanson N A, Jr, Glembotski C C, Steinhelper M E, Field L J, Claycomb W C. Circulation. 1992;85:1835–1841. - PubMed
-
- Borisov A B, Claycomb W C. Ann NY Acad Sci. 1995;752:80–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

