Engineering of plasmin-resistant forms of streptokinase and their production in Bacillus subtilis: streptokinase with longer functional half-life
- PMID: 9501422
- PMCID: PMC106333
- DOI: 10.1128/AEM.64.3.824-829.1998
Engineering of plasmin-resistant forms of streptokinase and their production in Bacillus subtilis: streptokinase with longer functional half-life
Abstract
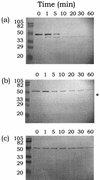
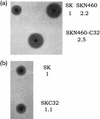
The short in vivo half-life of streptokinase limits its efficacy as an efficient blood clot-dissolving agent. During the clot-dissolving process, streptokinase is processed to smaller intermediates by plasmin. Two of the major processing sites are Lys59 and Lys386. We engineered two versions of streptokinase with either one of the lysine residues changed to glutamine and a third version with both mutations. These mutant streptokinase proteins (muteins) were produced by secretion with the protease-deficient Bacillus subtilis WB600 as the host. The purified muteins retained comparable kinetics parameters in plasminogen activation and showed different degrees of resistance to plasmin depending on the nature of the mutation. Muteins with double mutations had half-lives that were extended 21-fold when assayed in a 1:1 molar ratio with plasminogen in vitro and showed better plasminogen activation activity with time in the radial caseinolysis assay. This study indicates that plasmin-mediated processing leads to the inactivation of streptokinase and is not required to convert streptokinase to its active form. Plasmin-resistant forms of streptokinase can be engineered without affecting their activity, and blockage of the N-terminal cleavage site is essential to generate engineered streptokinase with a longer in vitro functional half-life.
Figures

References
-
- Bajaj A P, Castellino F J. Activation of human plasminogen by equimolar levels of streptokinase. J Biol Chem. 1977;252:492–498. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Breton J, Pezzi N, Molinari A, Bonomini L, Lansen J, Gonzalez De Buitrago G, Prieto I. Prolonged half-life in the circulation of a chemical conjugate between a pro-urokinase derivative and human serum albumin. Eur J Biochem. 1995;231:563–569. - PubMed
-
- Brockway W J, Castellino F J. A characterization of native streptokinase and altered streptokinase isolated from a human plasminogen activator complex. Biochemistry. 1974;13:2063–2070. - PubMed
-
- Brucato F H, Pizzo S V. Catabolism of streptokinase and polyethylene glycol-streptokinase: evidence for transport of intact forms through the biliary system in the mouse. Blood. 1990;76:73–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

