Expression of Clostridium acetobutylicum ATCC 824 genes in Escherichia coli for acetone production and acetate detoxification
- PMID: 9501448
- PMCID: PMC106371
- DOI: 10.1128/AEM.64.3.1079-1085.1998
Expression of Clostridium acetobutylicum ATCC 824 genes in Escherichia coli for acetone production and acetate detoxification
Abstract
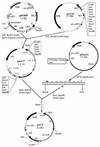
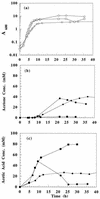
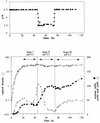
A synthetic acetone operon (ace4) composed of four Clostridium acetobutylicum ATCC 824 genes (adc, ctfAB, and thl, coding for the acetoacetate decarboxylase, coenzyme A transferase, and thiolase, respectively) under the control of the thl promoter was constructed and was introduced into Escherichia coli on vector pACT. Acetone production demonstrated that ace4 is expressed in E. coli and resulted in the reduction of acetic acid levels in the fermentation broth. Since different E. coli strains vary significantly in their growth characteristics and acetate metabolism, ace4 was expressed in three E. coli strains: ER2275, ATCC 11303, and MC1060. Shake flask cultures of MC1060(pACT) produced ca. 2 mM acetone, while both strains ER2275(pACT) and ATCC 11303(pACT) produced ca. 40 mM acetone. Glucose-fed cultures of strain ATCC 11303(pACT) resulted in a 150% increase in acetone titers compared to those of batch shake flask cultures. External addition of sodium acetate to glucose-fed cultures of ATCC 11303(pACT) resulted in further increased acetone titers. In bioreactor studies, acidic conditions (pH 5.5 versus 6.5) improved acetone production. Despite the substantial acetone evaporation due to aeration and agitation in the bioreactor, 125 to 154 mM acetone accumulated in ATCC 11303(pACT) fermentations. These acetone titers are equal to or higher than those produced by wild-type C. acetobutylicum. This is the first study to demonstrate the ability to use clostridial genes in nonclostridial hosts for solvent production. In addition, acetone-producing E. coli strains may be useful hosts for recombinant protein production in that detrimental acetate accumulation can be avoided.
Figures
References
-
- Aristidou A A, San K-Y, Bennett G N. Modification of central metabolic pathway in Escherichia coli to reduce acetate accumulation by heterologous expression of the Bacillus subtilis acetolactate synthase gene. Biotechnol Bioeng. 1994;44:944–951. - PubMed
-
- Aristidou A A, San K-Y, Bennett G N. Metabolic engineering of Escherichia coli to enhance recombinant protein production through acetate reduction. Biotechnol Prog. 1995;11:475–478. - PubMed
-
- Bennett G N, Rudolph F B. The central metabolic pathway from acetyl-CoA to butyryl-CoA in Clostridium acetobutylicum. FEMS Microbiol Rev. 1995;17:241–249.
-
- Bermejo Martinez L L. Heterologous expression of Clostridium acetobutylicum genes in Escherichia coli for acetone production. M.S. thesis. Evanston, Ill: Northwestern University; 1996. - PubMed
-
- Clark D P. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;63:223–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

