Upregulation of L-type Ca2+ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia
- PMID: 9502793
- PMCID: PMC6793103
- DOI: 10.1523/JNEUROSCI.18-07-02321.1998
Upregulation of L-type Ca2+ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia
Abstract
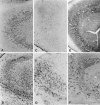
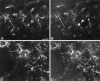
Anti-peptide antibodies that specifically recognize the alpha1 subunit of class A-D voltage-gated Ca2+ channels and a monoclonal antibody (MANC-1) to the alpha2 subunit of L-type Ca2+ channels were used to investigate the distribution of these Ca2+ channel subtypes in neurons and glia in models of brain injury, including kainic acid-induced epilepsy in the hippocampus, mechanical and thermal lesions in the forebrain, hypomyelination in white matter, and ischemia. Immunostaining of the alpha2 subunit of L-type Ca2+ channels by the MANC-1 antibody was increased in reactive astrocytes in each of these forms of brain injury. The alpha1C subunits of class C L-type Ca2+ channels were upregulated in reactive astrocytes located in the affected regions in each of these models of brain injury, although staining for the alpha1 subunits of class D L-type, class A P/Q-type, and class B N-type Ca2+ channels did not change from patterns normally observed in control animals. In all of these models of brain injury, there was no apparent redistribution or upregulation of the voltage-gated Ca2+ channels in neurons. The upregulation of L-type Ca2+ channels in reactive astrocytes may contribute to the maintenance of ionic homeostasis in injured brain regions, enhance the release of neurotrophic agents to promote neuronal survival and differentiation, and/or enhance signaling in astrocytic networks in response to injury.
Figures







Similar articles
-
Enhanced expression of L-type Ca2+ channels in reactive astrocytes after ischemic injury in rats.Neurosci Lett. 2001 Apr 20;302(2-3):93-6. doi: 10.1016/s0304-3940(01)01683-4. Neurosci Lett. 2001. PMID: 11290395
-
Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ.J Neurosci. 1998 Sep 15;18(18):7232-43. doi: 10.1523/JNEUROSCI.18-18-07232.1998. J Neurosci. 1998. PMID: 9736645 Free PMC article.
-
Activated astrocytes in areas of kainate-induced neuronal injury upregulate the expression of the metabotropic glutamate receptors 2/3 and 5.Exp Brain Res. 2001 Mar;137(1):1-11. doi: 10.1007/s002210000633. Exp Brain Res. 2001. PMID: 11310162
-
Altered Homeostatic Functions in Reactive Astrocytes and Their Potential as a Therapeutic Target After Brain Ischemic Injury.Curr Pharm Des. 2017;23(33):5056-5074. doi: 10.2174/1381612823666170710161858. Curr Pharm Des. 2017. PMID: 28699523 Review.
-
[Cerebral ischemia-hypoxia and biophysical mechanisms of neurodegeneration and neuroprotection effects].Fiziol Zh (1994). 2003;49(2):7-12. Fiziol Zh (1994). 2003. PMID: 12945108 Review. Ukrainian.
Cited by
-
Calmodulin kinase pathway mediates the K+-induced increase in Gap junctional communication between mouse spinal cord astrocytes.J Neurosci. 2001 Sep 1;21(17):6635-43. doi: 10.1523/JNEUROSCI.21-17-06635.2001. J Neurosci. 2001. PMID: 11517253 Free PMC article.
-
Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity.J Biol Chem. 2008 Aug 8;283(32):21953-64. doi: 10.1074/jbc.M800148200. Epub 2008 Jun 9. J Biol Chem. 2008. PMID: 18541537 Free PMC article.
-
Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention.Neural Regen Res. 2024 Nov 1;19(11):2480-2487. doi: 10.4103/1673-5374.390966. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38526284 Free PMC article.
-
The inhibitory neurotransmitter GABA evokes long-lasting Ca(2+) oscillations in cortical astrocytes.Glia. 2016 Mar;64(3):363-73. doi: 10.1002/glia.22933. Epub 2015 Oct 23. Glia. 2016. PMID: 26496414 Free PMC article.
-
L-type voltage-operated calcium channels contribute to astrocyte activation In vitro.Glia. 2016 Aug;64(8):1396-415. doi: 10.1002/glia.23013. Epub 2016 Jun 1. Glia. 2016. PMID: 27247164 Free PMC article.
References
-
- Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive Ca2+ channels in mammalian brain, spinal cord, and retina. Neuron. 1990;4:819–832. - PubMed
-
- Aicardi G, Schwartzkroin PA. Suppression of epileptiform burst discharges in CA1 neurons of rat hippocampal slices by the organic Ca2+ channel blocker, verapamil. Exp Brain Res. 1990;81:288–296. - PubMed
-
- Andersson H, Luthman J, Oslon L. Trimethyltin-induced expression of GABA and vimentin immunoreactivities in astrocytes of the rat brain. Glia. 1994;11:378–382. - PubMed
-
- Astrup J, Norberg K. K+ activity in cerebral cortex in rats during progressive severe hypoglycemia. Brain Res. 1976;103:418–423. - PubMed
-
- Ballarin M, Ernfors P, Linderfors N, Persson H. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol. 1991;114:35–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
