Honeybee blue- and ultraviolet-sensitive opsins: cloning, heterologous expression in Drosophila, and physiological characterization
- PMID: 9502802
- PMCID: PMC6793122
- DOI: 10.1523/JNEUROSCI.18-07-02412.1998
Honeybee blue- and ultraviolet-sensitive opsins: cloning, heterologous expression in Drosophila, and physiological characterization
Abstract
The honeybee (Apis mellifera) visual system contains three classes of retinal photoreceptor cells that are maximally sensitive to light at 440 nm (blue), 350 nm (ultraviolet), and 540 nm (green). We performed a PCR-based screen to identify the genes encoding the Apis blue- and ultraviolet (UV)-sensitive opsins. We obtained cDNAs that encode proteins having a high degree of sequence and structural similarity to other invertebrate and vertebrate visual pigments. The Apis blue opsin cDNA encodes a protein of 377 amino acids that is most closely related to other invertebrate visual pigments that are thought to be blue-sensitive. The UV opsin cDNA encodes a protein of 371 amino acids that is most closely related to the UV-sensitive Drosophila Rh3 and Rh4 opsins. To test whether these novel Apis opsin genes encode functional visual pigments and to determine their spectral properties, we expressed them in the R1-6 photoreceptor cells of blind ninaE mutant Drosophila, which lack the major opsin of the fly compound eye. We found that the expression of either the Apis blue- or UV-sensitive opsin in transgenic flies rescued the visual defect of ninaE mutants, indicating that both genes encode functional visual pigments. Spectral sensitivity measurements of these flies demonstrated that the blue and UV visual pigments are maximally sensitive to light at 439 and 353 nm, respectively. These maxima are in excellent agreement with those determined previously by single-cell recordings from Apis photoreceptor cells and provide definitive evidence that the genes described here encode visual pigments having blue and UV sensitivity.
Figures




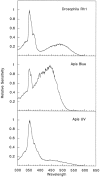
Similar articles
-
Characterisation of the ultraviolet-sensitive opsin gene in the honey bee, Apis mellifera.Eur J Biochem. 1997 Feb 1;243(3):775-81. doi: 10.1111/j.1432-1033.1997.00775.x. Eur J Biochem. 1997. PMID: 9057845
-
Primary structure of locust opsins: a speculative model which may account for ultraviolet wavelength light detection.Vision Res. 1997 Mar;37(5):495-503. doi: 10.1016/s0042-6989(96)00198-8. Vision Res. 1997. PMID: 9156194
-
Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: visual physiology and photochemistry of transgenic animals.J Neurosci. 1992 Oct;12(10):3862-8. doi: 10.1523/JNEUROSCI.12-10-03862.1992. J Neurosci. 1992. PMID: 1403087 Free PMC article.
-
Reconstructing the ancestral butterfly eye: focus on the opsins.J Exp Biol. 2008 Jun;211(Pt 11):1805-13. doi: 10.1242/jeb.013045. J Exp Biol. 2008. PMID: 18490396 Review.
-
Molecular evolution of vertebrate visual pigments.Prog Retin Eye Res. 2000 Jul;19(4):385-419. doi: 10.1016/s1350-9462(00)00002-1. Prog Retin Eye Res. 2000. PMID: 10785616 Review.
Cited by
-
Jewel Beetle Opsin Duplication and Divergence Is the Mechanism for Diverse Spectral Sensitivities.Mol Biol Evol. 2023 Feb 3;40(2):msad023. doi: 10.1093/molbev/msad023. Mol Biol Evol. 2023. PMID: 36721951 Free PMC article.
-
Molecular advances to study the function, evolution and spectral tuning of arthropod visual opsins.Philos Trans R Soc Lond B Biol Sci. 2022 Oct 24;377(1862):20210279. doi: 10.1098/rstb.2021.0279. Epub 2022 Sep 5. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36058235 Free PMC article. Review.
-
Coexpression of two visual pigments in a photoreceptor causes an abnormally broad spectral sensitivity in the eye of the butterfly Papilio xuthus.J Neurosci. 2003 Jun 1;23(11):4527-32. doi: 10.1523/JNEUROSCI.23-11-04527.2003. J Neurosci. 2003. PMID: 12805293 Free PMC article.
-
Dynamin- and Rab5-dependent endocytosis is required to prevent Drosophila photoreceptor degeneration.J Cell Sci. 2011 May 1;124(Pt 9):1564-70. doi: 10.1242/jcs.082115. Epub 2011 Apr 12. J Cell Sci. 2011. PMID: 21486953 Free PMC article.
-
Molecular Evolution of Malacostracan Short Wavelength Sensitive Opsins.J Mol Evol. 2023 Dec;91(6):806-818. doi: 10.1007/s00239-023-10137-w. Epub 2023 Nov 9. J Mol Evol. 2023. PMID: 37940679
References
-
- Asenjo AB, Rim J, Oprian DD. Molecular determinants of human red/green color discrimination. Neuron. 1994;12:1131–1138. - PubMed
-
- Bellingham J, Wilkie SE, Morris AG, Bowmaker JK, Hunt DM. Characterisation of the ultraviolet-sensitive opsin gene in the honey bee, Apis mellifera. Eur J Biochem. 1997;243:775–781. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
