Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors
- PMID: 9502803
- PMCID: PMC6793094
- DOI: 10.1523/JNEUROSCI.18-07-02423.1998
Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors
Abstract
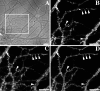
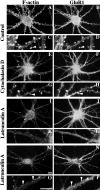
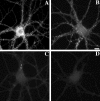
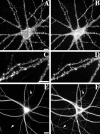
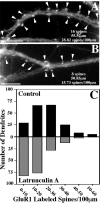
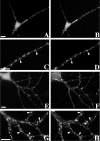
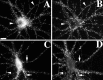
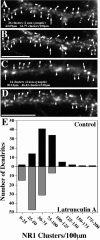
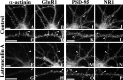
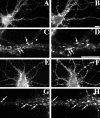
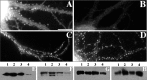
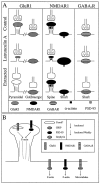
We used actin-perturbing agents and detergent extraction of primary hippocampal cultures to test directly the role of the actin cytoskeleton in localizing GABAA receptors, AMPA- and NMDA-type glutamate receptors, and potential anchoring proteins at postsynaptic sites. Excitatory postsynaptic sites on dendritic spines contained a high concentration of F-actin that was resistant to cytochalasin D but could be depolymerized using the novel compound latrunculin A. Depolymerization of F-actin led to a 40% decrease in both the number of synaptic NMDA receptor (NMDAR1) clusters and the number of AMPA receptor (GluR1)-labeled spines. The nonsynaptic NMDA receptors appeared to remain clustered and to coalesce in cell bodies. alpha-Actinin-2, which binds both actin and NMDA receptors, dissociated from the receptor clusters, but PSD-95 remained associated with both the synaptic and nonsynaptic receptor clusters, consistent with a proposed cross-linking function. AMPA receptors behaved differently; on GABAergic neurons, the clusters redistributed to nonsynaptic sites, whereas on pyramidal neurons, many of the clusters appeared to disperse. Furthermore, in control neurons, AMPA receptors were detergent extractable from pyramidal cell spines, whereas AMPA receptors on GABAergic neurons and NMDA receptors were unextractable. GABAA receptors were not dependent on F-actin for the maintenance or synaptic localization of clusters. These results indicate fundamental differences in the mechanisms of receptor anchoring at postsynaptic sites, both regarding the anchoring of a single receptor (the AMPA receptor) in pyramidal cells versus GABAergic interneurons and regarding the anchoring of different receptors (AMPA vs NMDA receptors) at a single class of postsynaptic sites on pyramidal cell dendritic spines.
Figures
References
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
-
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
-
- Beck KA, Nelson WJ. The spectrin-based membrane skeleton as a membrane protein-sorting machine. Am J Physiol. 1996;270:C1263–C1270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
