Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study
- PMID: 9502804
- PMCID: PMC6793096
- DOI: 10.1523/JNEUROSCI.18-07-02437.1998
Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study
Abstract
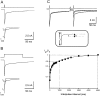
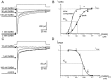
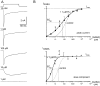
Although GABA type A receptors (GABAARs) in principal cells have been studied in detail, there is only limited information about GABAARs in interneurons. We have used the patch-clamp technique in acute rat hippocampal slices in combination with single-cell PCR to determine kinetic, pharmacological, and structural properties of dentate gyrus basket cell GABAARs. Application of 1 mM GABA (100 msec) to nucleated patches via a piezo-driven fast application device resulted in a current with a fast rise and a marked biexponential decay (time constants 2.4 and 61.8 msec). This decay could be attributed to strong receptor desensitization. Dose-response curves for the peak and the slow component yielded EC50 values of 139 and 24 microM, respectively. Zn2+ caused a marked blocking effect on both the peak and the slow component via a noncompetitive mechanism (IC50 values of 8 and 16 microM). This led to an acceleration of the slow component as well as a prolongation of recovery from desensitization. Zn2+ sensitivity was suggested to depend on the absence of gamma-subunits in GABAARs. To test this hypothesis we performed single-cell reverse transcription PCR that revealed primarily the presence of alpha2-, beta2-, beta3-, gamma1-, and gamma2-subunit mRNAs. In addition, flunitrazepam increased the receptor affinity for its agonist, indicating the presence of functional benzodiazepine binding sites, i.e., gamma-subunits. Thus, additional factors seem to co-determine the Zn2+ sensitivity of native GABAARs. The modulatory effects of Zn2+ on GABAAR desensitization suggest direct influences on synaptic integration via changes in inhibition and shunting at GABAergic synapses.
Figures


References
-
- Assaf YS, Chung S-H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. - PubMed
-
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. - PubMed
-
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
