Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma
- PMID: 9502808
- PMCID: PMC6793087
- DOI: 10.1523/JNEUROSCI.18-07-02475.1998
Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma
Abstract
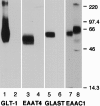
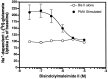
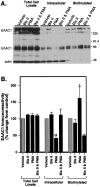
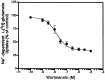
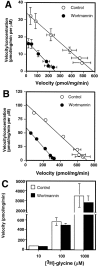
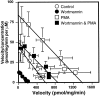
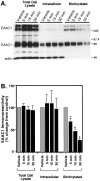
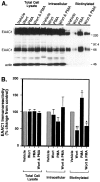
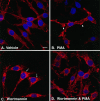
Neuronal and glial sodium-dependent transporters are crucial for the control of extracellular glutamate levels in the CNS. The regulation of these transporters is relatively unexplored, but the activity of other transporters is regulated by protein kinase C (PKC)- and phosphatidylinositol 3-kinase (PI3K)-mediated trafficking to and from the cell surface. In the present study the C6 glioma cell line was used as a model system that endogenously expresses the excitatory amino acid carrier 1 (EAAC1) subtype of neuronal glutamate transporter. As previously observed, phorbol 12-myristate 13-acetate (PMA) caused an 80% increase in transporter activity within minutes that cannot be attributed to the synthesis of new transporters. This increase in activity correlated with an increase in cell surface expression of EAAC1 as measured by using a membrane-impermeant biotinylation reagent. Both effects of PMA were blocked by the PKC inhibitor bisindolylmaleimide II (Bis II). The putative PI3K inhibitor, wortmannin, decreased L-[3H]-glutamate uptake activity by >50% within minutes. Wortmannin decreased the Vmax of L-[3H]-glutamate and D-[3H]-aspartate transport, but it did not affect Na+-dependent [3H]-glycine transport. Wortmannin also decreased cell surface expression of EAAC1. Although wortmannin did not block the effects of PMA on activity, it prevented the PMA-induced increase in cell surface expression. This trafficking of EAAC1 also was examined with immunofluorescent confocal microscopy, which supported the biotinylation studies and also revealed a clustering of EAAC1 at cell surface after treatment with PMA. These studies suggest that the trafficking of the neuronal glutamate transporter EAAC1 is regulated by two independent signaling pathways and also may suggest a novel endogenous protective mechanism to limit glutamate-induced excitotoxicity.
Figures

References
-
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. - PubMed
-
- Casado M, Bendahan A, Zafra F, Danbolt NC, Gimenez C, Kanner BI. Phosphorylation and modulation of brain glutamate transporters by protein kinase C. J Biol Chem. 1993;268:27313–27317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
