Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes
- PMID: 9502811
- PMCID: PMC6793100
- DOI: 10.1523/JNEUROSCI.18-07-02506.1998
Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes
Abstract
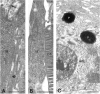
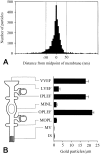
The water permeability of cell membranes differs by orders of magnitude, and most of this variability reflects the differential expression of aquaporin water channels. We have recently found that the CNS contains a member of the aquaporin family, aquaporin-4 (AQP4). As a prerequisite for understanding the cellular handling of water during neuronal activity, we have investigated the cellular and subcellular expression of AQP4 in the retina and optic nerve where activity-dependent ion fluxes have been studied in detail. In situ hybridization with digoxigenin-labeled riboprobes and immunogold labeling by a sensitive postembedding procedure demonstrated that AQP4 and AQP4 mRNA were restricted to glial cells, including MHller cells in the retina and fibrous astrocytes in the optic nerve. A quantitative immunogold analysis of the MHller cells showed that these cells exhibited three distinct membrane compartments with regard to AQP4 expression. End feet membranes (facing the vitreous body or blood vessels) were 10-15 times more intensely labeled than non-end feet membranes, whereas microvilli were devoid of AQP4. These data suggest that MHller cells play a prominent role in the water handling in the retina and that they direct osmotically driven water flux to the vitreous body and vessels rather than to the subretinal space. Fibrous astrocytes in the optic nerve similarly displayed a differential compartmentation of AQP4. The highest expression of AQP4 occurred in end feet membranes, whereas the membrane domain facing the nodal axolemma was associated with a lower level of immunoreactivity than the rest of the membrane. This arrangement may allow transcellular water redistribution to occur without inducing inappropriate volume changes in the perinodal extracellular space.
Figures






References
-
- Amédée T, Robert A, Coles JA. Potassium homeostasis and glial energy metabolism. Glia. 1997;21:46–55. - PubMed
-
- Andrew RD, MacVicar BA. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994;62:371–383. - PubMed
-
- Brew H, Gray PTA, Mobbs P, Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering. Nature. 1986;324:466–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
