Astrocytic gap junctions remain open during ischemic conditions
- PMID: 9502812
- PMCID: PMC6793088
- DOI: 10.1523/JNEUROSCI.18-07-02520.1998
Astrocytic gap junctions remain open during ischemic conditions
Abstract
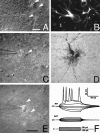
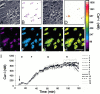
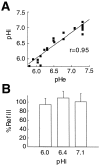
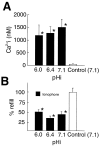
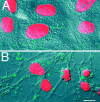
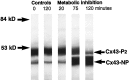
Gap junctions are highly conductive channels that allow the direct transfer of intracellular messengers such as Ca2+ and inositol triphosphate (IP3) between interconnected cells. In brain, astrocytes are coupled extensively by gap junctions. We found here that gap junctions among astrocytes in acutely prepared brain slices as well as in culture remained open during ischemic conditions. Uncoupling first occurred after the terminal loss of plasma membrane integrity. Gap junctions therefore may link ischemic astrocytes in an evolving infarct with the surroundings. The free exchange of intracellular messengers between dying and potentially viable astrocytes might contribute to secondary expansion of ischemic lesions.
Figures








References
-
- Anders JJ. Lactic acid inhibition of gap junctional intercellular communication in in vitro astrocytes as measured by fluorescence recovery after laser photobleaching. Glia. 1988;1:371–379. - PubMed
-
- Arellano G, Ramon F, Rivera A, Zampighi G. Calmodulin acts as an intermediary for the effects of calcium on gap junctions from crayfish lateral axons. J Membr Biol. 1988;101:119–131. - PubMed
-
- Astrup J, Symon L, Branston N, Lassen N. Cortical evoked potential and extracellular potassium and hydrogen at cortical levels of brain ischemia. Stroke. 1977;8:51–57. - PubMed
-
- Astrup J, Siesjo B, Symon L. Threshold in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
