Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning
- PMID: 9502818
- PMCID: PMC6793105
- DOI: 10.1523/JNEUROSCI.18-07-02592.1998
Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning
Abstract
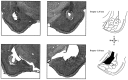
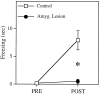
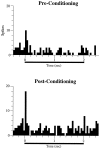
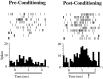
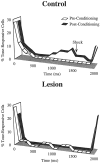
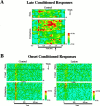
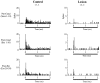
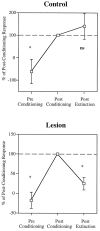
In auditory fear conditioning, pairing of a neutral acoustic conditioned stimulus (CS) with an aversive unconditioned stimulus (US) results in an enhancement of neural responses to the CS in the amygdala and auditory cortex. It is not clear, however, whether cortical plasticity governs neural changes in the amygdala or vice versa, or whether learning in these two structures is determined by independent processes. We examined this issue by recording single-cell activity in the auditory cortex (areas Te1, Te1v, and Te3) of freely behaving, amygdalectomized rats using a movable bundle of microwires. Amygdala damage did not affect short-latency (0-50 msec) tone responses, nor did it interfere with conditioning-induced increases of these onset responses. In contrast, lesions of the amygdala interfered with the development of late (500-1500 msec) conditioned tone responses that were not present before conditioning. Furthermore, whereas onset conditioned responses in the control group remained elevated after 30 extinction trials (presentation of CS alone), onset responses in lesioned animals returned to their preconditioning firing level after approximately 10 extinction trials. These results suggest that the amygdala enables the development of long-latency (US anticipatory) responses and prevents the extinction of short-latency onset responses to threatening stimuli. The findings further suggest that auditory cortex cells may participate differently in explicit and implicit memory networks.
Figures

References
-
- Amaral DG. In: Memory: anatomical organization of candidate brain regions. Handbook of physiology. Section 1: The nervous system. Plum F, editor. American Physiological Society; Bethesda, MD: 1987. pp. 211–294.
-
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 1–66.
-
- Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE. An anatomically constrained neural network model of fear conditioning. Behav Neurosci. 1995;109:246–257. - PubMed
-
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb Cortex. 1997a;7:157–165. - PubMed
-
- Armony JL, Servan-Schreiber D, Cohen JD, LeDoux JE. Computational modeling of emotion: explorations through the anatomy and physiology of fear conditioning. Trends Cognit Sci. 1997b;1:28–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
