Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons
- PMID: 9502823
- PMCID: PMC6793081
- DOI: 10.1523/JNEUROSCI.18-07-02653.1998
Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons
Abstract
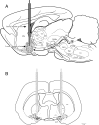
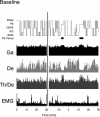
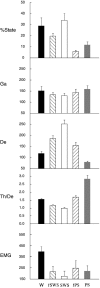
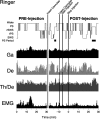
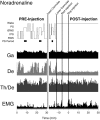
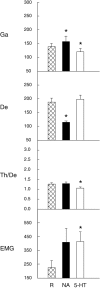
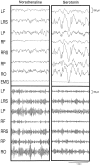
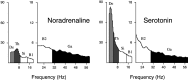
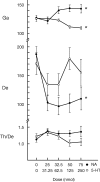
Several lines of evidence indicate that cholinergic basalis neurons play an important role in cortical activation. The present study was undertaken to determine the effect of noradrenergic and serotonergic modulation of the cholinergic neurons on cortical EEG activity and sleep-wake states. The neurotransmitters were injected into the region of the basalis neurons by remote control in freely moving, naturally sleeping-waking rats during the day when the rats are normally asleep the majority of the time. Effects were observed on behavior and EEG activity, including high-frequency gamma activity (30-60 Hz), which has been demonstrated to reflect behavioral and cortical arousal in the rat. Noradrenaline, which has been shown in previous in vitro studies to depolarize and excite the cholinergic cells, produced a dose-dependent increase in gamma-EEG activity, a decrease in delta activity, and an increase in waking. Serotonin, which has been found in previous in vitro studies to hyperpolarize the cholinergic neurons, produced a dose-dependent decrease in gamma-EEG activity with no significant change in amounts of wake or slow wave sleep. Both chemicals resulted in a dose-dependent decrease in paradoxical sleep. These results demonstrate that noradrenaline and serotonin exert differential modulatory effects on EEG activity through the basal forebrain, the one facilitating gamma activity and eliciting waking and the other diminishing gamma activity and not significantly affecting slow wave sleep. The results also confirm that the cholinergic basalis neurons play an important role in cortical activation and particularly in the high-frequency gamma activity that underlies cortical and behavioral arousal of the wake state.
Figures


References
-
- Aghajanian GK, Wang RY, Barban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 1978;153:169–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
