Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse
- PMID: 9502833
- PMCID: PMC6793092
- DOI: 10.1523/JNEUROSCI.18-07-02764.1998
Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse
Abstract
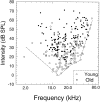
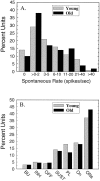
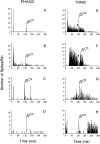
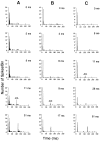
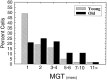
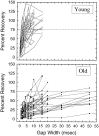
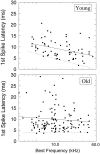
The perception of complex sounds, such as speech and animal vocalizations, requires the central auditory system to analyze rapid, ongoing fluctuations in sound frequency and intensity. A decline in temporal acuity has been identified as one component of age-related hearing loss. The detection of short, silent gaps is thought to reflect an important fundamental dimension of temporal resolution. In this study we compared the neural response elicited by silent gaps imbedded in noise of single neurons in the inferior colliculus (IC) of young and old CBA mice. IC neurons were classified by their temporal discharge patterns. Phasic units, which accounted for the majority of response types encountered, tended to have the shortest minimal gap thresholds (MGTs), regardless of age. We report three age-related changes in neural processing of silent gaps. First, although the shortest MGTs (1-2 msec) were observed in phasic units from both young and old animals, the number of neurons exhibiting the shortest MGTs was much lower in old mice, regardless of the presentation level. Second, in the majority of phasic units, recovery of response to the stimulus after the silent gap was of a lower magnitude and much slower in units from old mice. Finally, the neuronal map representing response latency versus best frequency was found to be altered in the old IC. These results demonstrate a central auditory system correlate for age-related decline in temporal processing at the level of the auditory midbrain.
Figures



References
-
- Adams JC. Ascending connections to the inferior colliculus. J Comp Neurol. 1979;183:519–538. - PubMed
-
- Aitkin L. The auditory brainstem. Integration and conclusions (Ottoson D, ed), pp 128–209. Springer; Berlin: 1986.
-
- Aitkin L, Tran L, Syka J. The responses of neurons in subdivisions of the inferior colliculus of cats to tonal, noise and vocal stimuli. Exp Brain Res. 1994;98:53–64. - PubMed
-
- Baimbridge KG, Celio MR, Roger JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–307. - PubMed
-
- Barsz K, Benson PK, Walton JP. Gap encoding by inferior collicular neurons is altered by minimal changes in signal envelope. Hear Res. 1998;115:13–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
