Coding of serial order by neostriatal neurons: a "natural action" approach to movement sequence
- PMID: 9502834
- PMCID: PMC6793101
- DOI: 10.1523/JNEUROSCI.18-07-02777.1998
Coding of serial order by neostriatal neurons: a "natural action" approach to movement sequence
Abstract
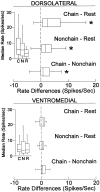
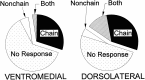
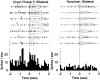
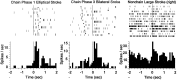
The neostriatum controls behavioral sequencing, or action syntax, as well as simpler aspects of movement. Yet the precise nature of the neostriatums role in sequencing remains unclear. Here we used a "natural action" approach that combined electrophysiological and neuroethological techniques. We identified neostriatal neurons that code the serial order of natural movement sequences of rats. During grooming behavior, rats emit complex but highly predictable species-specific sequences of movements, termed "syntactic chains." Neuronal activity of 41% of cells in the dorsolateral and ventromedial neostriatum coded the sequential pattern of syntactic chains. Only 14% coded simple motor properties of grooming movements. Neurons fired preferentially during syntactic chains compared with similar grooming movements made in different sequential order or to behavioral resting. Sequential coding differed between the dorsolateral and ventromedial neostriatum. Neurons in the dorsolateral site increased firing by 116% during syntactic chains, compared with only a 30% increase by neurons in the ventromedial site, and dorsolateral neurons showed strongest coding of grooming syntax by several additional criteria. These data demonstrate that neostriatal neurons code abstract properties of serial order for natural movement and support the hypothesis that the dorsolateral neostriatum plays a special role in implementing action syntax.
Figures






References
-
- Aldridge JW, Gilman S. The temporal structure of spike trains in the primate basal ganglia—afferent regulation of bursting demonstrated with precentral cerebral cortical ablation. Brain Res. 1991;543:123–138. - PubMed
-
- Aldridge JW, Anderson RJ, Murphy JT. Sensory motor processing in the caudate nucleus and globus pallidus: a single unit study in behaving primates. Can J Physiol Pharmacol. 1980a;58:1192–1201. - PubMed
-
- Aldridge JW, Anderson RJ, Murphy JT. The role of the basal ganglia in controlling a movement initiated by a visually presented cue. Brain Res. 1980b;192:3–16. - PubMed
-
- Aldridge JW, Berridge KC, Herman M, Zimmer L. Neuronal coding of serial order: syntax of grooming in the neostriatum. Psychol Sci. 1993;4:391–395.
-
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in 3 motor areas of the monkey. J Neurophysiol. 1990;64:133–150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
