In vivo modulation of interacting central pattern generators in lobster stomatogastric ganglion: influence of feeding and partial pressure of oxygen
- PMID: 9502835
- PMCID: PMC6793085
- DOI: 10.1523/JNEUROSCI.18-07-02788.1998
In vivo modulation of interacting central pattern generators in lobster stomatogastric ganglion: influence of feeding and partial pressure of oxygen
Abstract
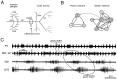
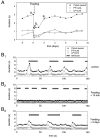
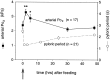
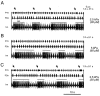
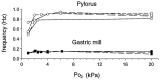
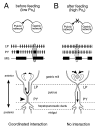
The stomatogastric ganglion (STG) of the European lobster Homarus gammarus contains two rhythm-generating networks (the gastric and pyloric circuits) that in resting, unfed animals produce two distinct, yet strongly interacting, motor patterns. By using simultaneous EMG recordings from the gastric and pyloric muscles in vivo, we found that after feeding, the gastropyloric interaction disappears as the two networks express accelerated motor rhythms. The return to control levels of network activity occurs progressively over the following 1-2 d and is associated with a gradual reappearance of the gastropyloric interaction. In parallel with this change in network activity is an alteration of oxygen levels in the blood. In resting, unfed animals, arterial partial pressure of oxygen (PO2) is most often between 1 and 2 kPa and then doubles within 1 hr after feeding, before returning to control values some 24 hr later. In vivo, experimental prevention of the arterial PO2 increase after feeding leads to a slowing of pyloric rhythmicity toward control values and a reappearance of the gastropyloric interaction, without apparent effect on gastric network operation. Using in vitro preparations of the stomatogastric nervous system and by changing oxygen levels uniquely at the level of the STG within the range observed in the intact animal, we were able to mimic most of the effects observed in vivo. Our data indicate that the gastropyloric interaction appears only during a "free run" mode of foregut activity and that the coordinated operation of multiple neural networks may be modulated by local changes in oxygenation.
Figures



References
-
- Aiken DE. Proecdysis, setal development, and molt predictions in the American lobster (Homarus americanus). J Fish Res Board Can. 1973;30:1337–1344.
-
- Barker PL, Gibson R. Observations on the feeding mechanism, structure of the gut, and digestive physiology of the European lobster Homarus gammarus. J Exp Mar Biol Ecol. 1977;26:297–324.
-
- Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. - PubMed
-
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
