Zn2+ inhibition of recombinant GABAA receptors: an allosteric, state-dependent mechanism determined by the gamma-subunit
- PMID: 9503325
- PMCID: PMC2230740
- DOI: 10.1111/j.1469-7793.1998.609bv.x
Zn2+ inhibition of recombinant GABAA receptors: an allosteric, state-dependent mechanism determined by the gamma-subunit
Abstract
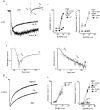
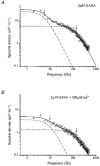
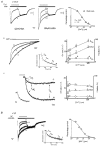
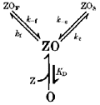
1. The gamma-subunit in recombinant gamma-aminobutyric acid (GABAA) receptors reduces the sensitivity of GABA-triggered Cl- currents to inhibition by Zn2+ and transforms the apparent mechanism of antagonism from non-competitive to competitive. To investigate underlying receptor function we studied Zn2- effects on macroscopic and single-channel currents of recombinant alpha 1 beta 2 and alpha 1 beta 2 gamma 2 receptors expressed heterologously in HEK-293 cells using the patch-clamp technique and rapid solution changes. 2. Zn2+ present for > 60 s (constant) inhibited peak, GABA (5 microM)-triggered currents of alpha 1 beta 2 receptors in a concentration-dependent manner (inhibition equation parameters: concentration at half-amplitude (IC50) = 0.94 microM; slope related to Hill coefficient, S = 0.7) that was unaffected by GABA concentration. The gamma 2 subunit (alpha 1 beta 2 gamma 2 receptor) reduced Zn2+ sensitivity more than fiftyfold (IC50 = 51 microM, S = 0.86); increased GABA concentration (100 microM) antagonized inhibition by reducing apparent affinity (IC50 = 322 microM, S = 0.79). Zn2+ slowed macroscopic gating of alpha 1 beta 2 receptors by inducing a novel slow exponential component in the activation time course and suppressing a fast component of control desensitization. For alpha 1 beta 2 gamma 2 receptors, Zn2+ accelerated a fast component of apparent desensitization. 3. Zn2+ preincubations lasting up to 10 s markedly increased current depression and activation slowing of alpha 1 beta 2 receptors, but had little effect on currents from alpha 1 beta 2 gamma 2 receptors. 4. Steady-state fluctuation analysis of macroscopic alpha 1 beta 2 gamma 2 currents (n = 5) resulted in control (2 microM GABA) power density spectra that were fitted by a sum of two Lorentzian functions (relaxation times: 37 +/- 5.6 and 1.41 +/- 0.15 ms, means +/- S.E.M.). Zn2+ (200 microM) reduced the total power almost sixfold and accelerated the slow (23 +/- 2.8 ms, P < 0.05) without altering the fast (1.40 +/- 0.16 ms) relaxation time. The ratio (fast/slow) of Lorentzian areas was increased by Zn2+ (control, 3.39 +/- 0.55; Zn2+, 4.9 +/- 0.37, P < 0.05). 5. Zn2+ (500 microM) depression of previously activated current amplitudes (% control) for alpha 1 beta 2 gamma 2 receptors was independent of GABA concentration (5 microM, 13.2 +/- 0.72%; 100 microM, 12.2 +/- 2.9%, P < 0.8, n = 5). Both onset and offset inhibition time courses were biexponential. Onset rates were enhanced by Zn2+ concentration. Inhibition onset was also biexponential for preactivated alpha 1 beta 2 receptors with current depression more than fourfold less sensitive (5 microM GABA, IC50 = 3.8 microM, S = 0.84) relative to that in constant Zn2+. 6. The results lead us to propose a general model of Zn2+ inhibition of GABAA receptors in which Zn2+ binds to a single extracellular site, induces allosteric receptor inhibition involving two non-conducting states, site affinity is state-dependent, and the features of state dependence are determined by the gamma-subunit.
Figures




References
-
- Aniksztejn L, Charlton G, Ben-Ari Y. Selective release of endogenous zinc from the hippocampal mossy fibers in situ. Brain Research. 1987;404:58–64. 10.1016/0006-8993(87)91355-2. - DOI - PubMed
-
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–738. - PubMed
-
- Burkat PM, Roberts W, Gingrich KJ. Gamma subunit influences the mechanism of zinc inhibition in recombinant GABAA receptors. Journal of Neuroscience. 1995;21:A850.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

