Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons
- PMID: 9503329
- PMCID: PMC2230754
- DOI: 10.1111/j.1469-7793.1998.665bv.x
Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons
Abstract
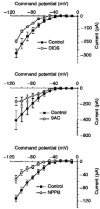
1. Dissociated rat superior cervical ganglion (SCG) neurons have been shown to possess a hyperpolarization-activated inwardly rectifying chloride current. The current was not altered by changes in external potassium concentration, replacing external cations with NMDG (N-methyl-D-glucamine) or by addition of 10 mM caesium or barium ions. 2. The reversal potential of the current was altered by changing external anions. The anion selectivity of the current was Cl- > Br- > I- > cyclamate. All substituted permeant anions also blocked the current. 3. The current was blocked by DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid), 9AC (anthracene-9-carboxylic acid) and NPPB (5-nitro-2-(3-phenylpropylamino)benzoic acid) but was unaffected by SITS (4-acetamido-4'-isothiocyanatostilbene- 2,2'-disulphonic acid) and niflumic acid. The effective blockers were voltage dependent; DIDS and NPPB were more effective at depolarized potentials while 9AC was more effective at hyperpolarized potentials. 4. The current was enhanced by extracellular acidification and reduced by extracellular alkalinization. Reducing external osmolarity was without effect in conventional whole-cell recording but enhanced current amplitude in those perforated-patch recordings where little current was evident in control external solution. 5. The current in SCG neurons was blocked by external cadmium and zinc. ClC-2 chloride currents expressed in Xenopus oocytes were also sensitive to block by these divalent ions and by DIDS but the sensitivity of ClC-2 to block by cadmium ions was lower than that of the current in SCG neurons. 6. Reverse transcriptase-polymerase chain reaction (RT-PCR) experiments showed the presence of mRNA for ClC-2 in SCG neurons but not in rat cerebellar granule cells which do not possess a hyperpolarization-activated Cl- current. 7. The data suggest that ClC-2 may be functionally expressed in rat SCG neurons. This current may play a role in regulating the internal chloride concentration in these neurons and hence their response to activation of GABAA receptors.
Figures







References
-
- Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. American Journal of Physiology. 1992;263:L1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

