Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro
- PMID: 9503336
- PMCID: PMC2230758
- DOI: 10.1111/j.1469-7793.1998.755bv.x
Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro
Abstract
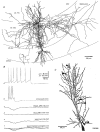
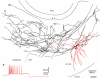
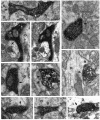
1. Hippocampal non-principal neurons at the stratum radiatum-stratum lacunosum-moleculare border (R-LM interneurons) of the CA1 area may constitute several cell classes and have been implicated in the generation of GABAergic unitary IPSPs. Using biocytin-filled electrodes we recorded R-LM interneurons intracellularly in vitro and determined their postsynaptic effects in concomitantly recorded pyramidal cells. 2. Light microscopic analysis revealed four populations of R-LM interneurons with distinct axons: (1) basket cells (n = 4) with axons predominantly ramifying in the pyramidal cell layer; (2) Schaffer collateral/commissural pathway-associated interneurons (n = 10) stratifying in stratum radiatum and, to a lesser extent, stratum oriens; (3) perforant pathway-associated interneurons (n = 6) innervating the perforant path termination zone in stratum lacunosum-moleculare of the CA1 area as well as equivalent portions of the dentate gyrus and subiculum; and (4) neurogliaform interneurons (n = 2) characterized by their dense, compact axonal and dendritic arbour. 3. Random electron microscopic sampling of synaptic targets revealed a preponderance of pyramidal neurons as postsynaptic elements. Basket cells had a synaptic target preference for somata and proximal dendrites, whereas the remainder of R-LM interneurons innervated dendritic shafts and spines. The axon of dendrite-targeting cells formed up to six putative contacts with individual postsynatpic pyramidal cells. 4. Anatomically recovered R-LM interneurons (n = 22) had a mean resting membrane potential of -56.7 +/- 3.6 mV, a membrane time constant of 12.9 +/- 7.7 ms and an input resistance of 86.4 +/- 29.2 M omega. Depolarizing current pulses generally elicited overshooting action potentials (70.8 +/- 6.9 mV) which had a mean duration, when measured at half-amplitude, of 0.7 +/- 0.1 ms. In response to prolonged (> 200 ms) depolarizing current pulses all R-LM interneurons displayed (a varying degree of) spike frequency adaptation. 5. Basket cells, Schaffer-associated and neurogliaform interneurons elicited small-amplitude (< 2 mV), short-latency IPSPs in postsynaptic pyramids (n = 5, 13 and 1, respectively). Those interactions in which an effect was elicited with the repetitive activation of the presynaptic neuron (n = 13) showed a substantial degree of postsynaptic response summation. Unitary IPSPs had fast kinetics and, whenever tested (n = 5; 1 basket cell and 4 Schaffer-associated interneurons), were abolished by the GABAA receptor antagonist bicuculline. 6. Thus, R-LM interneurons comprise several distinct populations which evoke fast GABAA receptor mediated IPSPs. The domain-specific innervation of postsynaptic pyramidal cells suggests functionally diverse effects on the integration of afferent information in functionally non-equivalent compartments of pyramidal cells.
Figures





References
-
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
-
- Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. European Journal of Neuroscience. 1995;7:1989–2004. - PubMed
-
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

